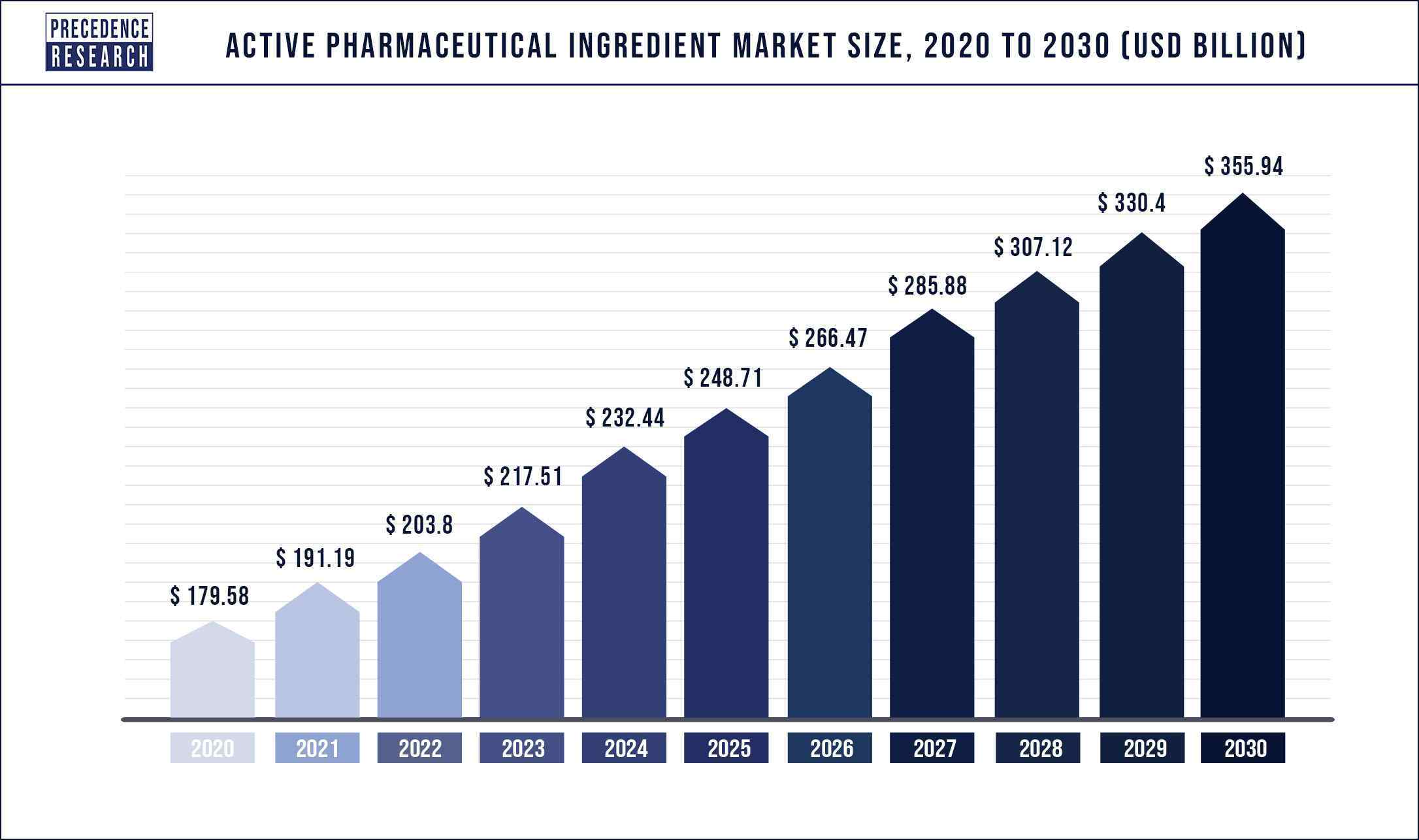
According to Precedence Research, during the forecast period of 2022 to 2030, the global active pharmaceutical ingredients market is estimated to develop at a compound annual growth rate (CAGR) of 7..1%. The global active pharmaceutical ingredients market was valued at USD 179.58 billion in 2021, and it is predicted to exceed USD 355.94 billion by 2030. The study investigates several elements and their consequences on the growth of the active pharmaceutical ingredients market.
Download Free Sample Copy with TOC@ https://www.precedenceresearch.com/sample/1035
Growth Factor
Continuously growing prevalence of chronic illness, escalating significance of generics, and the cumulative uptake of biopharmaceuticals are some of the prime factors stimulating the growth of the active pharmaceutical ingredient market globally. Alternatively, the critical drug price control policies in numerous nations and the swelling diffusion of counterfeit drugs may hamper the growth of API market in these nations during next few years. As per, 2017 NCBI article on a survey directed in a tertiary hospital in Japan, about 339 bacteremia UTI cases were identified. Thus, escalating occurrence of such infectious sickness and hospital-acquired contaminations are projected to stimulate growth of API market in the nearby future.
Some of the prime factors spurring the growth of the market are increasing occurrences of cardiovascular, oncology, lifestyle and diabetes diseases, intensifying number of diagnostic centers and hospitals and escalating elderly population in emerging countries. Furthermore, cumulative healthcare spending, growing disease responsiveness and education are propelling the growth of the market. Conversely, a shortage of accomplished workforce and inadequate accessibility of drugs in the emerging nations are hampering the growth of the market in these regions.
Ask Here For More Customization Study@ https://www.precedenceresearch.com/customization/1035
This report focuses on active pharmaceutical ingredients market volume and value at the global level, regional level and company level. From a global perspective, this report represents overall active pharmaceutical ingredients market size by analysing historical data and future prospect. Regionally, this report focuses on several key regions: North America, Europe, Middle East & Africa, Latin America, etc.
The research report includes specific segments by region (country), by company, by all segments. This study provides information about the growth and revenue during the historic and forecasted period of 2017 to 2030. Understanding the segments helps in identifying the importance of different factors that aid the market growth.
Report Highlights
- Some of the tactical initiatives commenced by many business players to sustain stability in the market are biological products, new drugs launches, collaborations, acquisitions, and geographical expansion.
- Oncology is projected to be the fastest-growing segment driven by developing pervasiveness of cancer and cumulative lifestyle-associated sicknesses.
- In the event of outsourcing, APIs are subject to strict regulations and omission from the nation they are shipped to. For example, API manufacturing plants abroad still go via an scrutiny by the U.S. Food & Drug Administration.
- API market has observed marvelous growth since last few decades due to augmented application of biologics and drugs in the treatment of diseases.
In-Depth Analysis on Competitive Landscape
The report sheds light on leading manufacturers of active pharmaceutical ingredients, along with their detailed profiles. Essential and up-to-date data related to market performers who are principally engaged in the production of active pharmaceutical ingredients has been brought with the help of a detailed dashboard view. Market share analysis and comparison of prominent players provided in the report permits report readers to take preemptive steps in advancing their businesses.
Company profiles have been included in the report, which include essentials such as product portfolio, key strategies, along with all-inclusive SWOT analysis on each player. Company presence is mapped and presented through a matrix for all the prominent players, thus providing readers with actionable insights, which helps in thoughtfully presenting market status and predicting the competition level in the active pharmaceutical ingredients market.
Some of the prominent players in the active pharmaceutical ingredients market include:
- Albemarle Corporation
- AurobindoPharma
- Reddy’s Laboratories Ltd.
- AbbVieInc
- Teva Pharmaceutical Industries Ltd
- Mylan N.V.
- CiplaInc
- BoehringerIngelheim International GmbH
- Merck & Co., Inc
- Sun Pharmaceutical Industries Ltd
- Bristol-Myers Squibb Company
Segments Covered in the Report
By Type of Manufacturer
- Merchant APIs
- Captive APIs
By Type
- Generic APIs
- Innovative APIs
By Type of Synthesis
- Biotech
- Recombinant Proteins
- Monoclonal Antibodies
- Vaccines
- Synthetic
By Application
- Orthopedic
- Pulmonology
- Gastroenterology
- Endocrinology
- Cardiology
- Oncology
- CNS & Neurology
- Nephrology
- Ophthalmology
- Others
Regional Segmentation
- Asia-Pacific [China, Southeast Asia, India, Japan, Korea, Western Asia]
- Europe [Germany, UK, France, Italy, Russia, Spain, Netherlands, Turkey, Switzerland]
- North America [United States, Canada, Mexico]
- South America [Brazil, Argentina, Columbia, Chile, Peru]
- Middle East & Africa [GCC, North Africa, South Africa]
Some of the important ones are:
- What can be the best investment choices for venturing into new product and service lines?
- What value propositions should businesses aim at while making new research and development funding?
- Which regulations will be most helpful for stakeholders to boost their supply chain network?
- Which regions might see the demand maturing in certain segments in near future?
- What are the some of the best cost optimization strategies with vendors that some well-entrenched players have gained success with?
- Which are the key perspectives that the C-suite are leveraging to move businesses to new growth trajectory?
- Which government regulations might challenge the status of key regional markets?
- How will the emerging political and economic scenario affect opportunities in key growth areas?
- What are some of the value-grab opportunities in various segments?
- What will be the barrier to entry for new players in the market?
Table of Contents
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. Market Dynamics Analysis and Trends
5.1. Market Dynamics
5.1.1. Market Drivers
5.1.2. Market Restraints
5.1.3. Market Opportunities
5.2. Porter’s Five Forces Analysis
5.2.1. Bargaining power of suppliers
5.2.2. Bargaining power of buyers
5.2.3. Threat of substitute
5.2.4. Threat of new entrants
5.2.5. Degree of competition
Chapter 6. Competitive Landscape
6.1.1. Company Market Share/Positioning Analysis
6.1.2. Key Strategies Adopted by Players
6.1.3. Vendor Landscape
6.1.3.1. List of Suppliers
6.1.3.2. List of Buyers
Chapter 7. Global Active Pharmaceutical Ingredient Market, By Type of Manufacturer
7.1. Active Pharmaceutical Ingredient Market, by Type of Manufacturer Type, 2021-2030
7.1.1. Merchant APIs
7.1.1.1. Market Revenue and Forecast (2019-2030)
7.1.2. Captive APIs
7.1.2.1. Market Revenue and Forecast (2019-2030)
Chapter 8. Global Active Pharmaceutical Ingredient Market, By Type
8.1. Active Pharmaceutical Ingredient Market, by Type, 2021-2030
8.1.1. Generic APIs
8.1.1.1. Market Revenue and Forecast (2019-2030)
8.1.2. Innovative APIs
8.1.2.1. Market Revenue and Forecast (2019-2030)
Chapter 9. Global Active Pharmaceutical Ingredient Market, By Type of Synthesis
9.1. Active Pharmaceutical Ingredient Market, by Type of Synthesis, 2021-2030
9.1.1. Biotech (Recombinant Proteins, Monoclonal Antibodies, Vaccines)
9.1.1.1. Market Revenue and Forecast (2019-2030)
9.1.2. Synthetic
9.1.2.1. Market Revenue and Forecast (2019-2030)
Chapter 10. Global Active Pharmaceutical Ingredient Market, By Application
10.1. Active Pharmaceutical Ingredient Market, by Application, 2021-2030
10.1.1. Orthopedic
10.1.1.1. Market Revenue and Forecast (2019-2030)
10.1.2. Pulmonology
10.1.2.1. Market Revenue and Forecast (2019-2030)
10.1.3. Gastroenterology
10.1.3.1. Market Revenue and Forecast (2019-2030)
10.1.4. Endocrinology
10.1.4.1. Market Revenue and Forecast (2019-2030)
10.1.5. Cardiology
10.1.5.1. Market Revenue and Forecast (2019-2030)
10.1.6. Oncology
10.1.6.1. Market Revenue and Forecast (2019-2030)
10.1.7. CNS & Neurology
10.1.7.1. Market Revenue and Forecast (2019-2030)
10.1.8. Nephrology
10.1.8.1. Market Revenue and Forecast (2019-2030)
10.1.9. Other
10.1.9.1. Market Revenue and Forecast (2019-2030)
Chapter 11. Global Active Pharmaceutical Ingredient Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.1.2. Market Revenue and Forecast, by Type (2019-2030)
11.1.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.1.4. Market Revenue and Forecast, by Application (2019-2030)
11.1.5. U.S.
11.1.5.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.1.5.2. Market Revenue and Forecast, by Type (2019-2030)
11.1.5.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.1.5.4. Market Revenue and Forecast, by Application (2019-2030)
11.1.6. Rest of North America
11.1.6.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.1.6.2. Market Revenue and Forecast, by Type (2019-2030)
11.1.6.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.1.6.4. Market Revenue and Forecast, by Application (2019-2030)
11.2. Europe
11.2.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.2.2. Market Revenue and Forecast, by Type (2019-2030)
11.2.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.2.4. Market Revenue and Forecast, by Application (2019-2030)
11.2.5. UK
11.2.5.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.2.5.2. Market Revenue and Forecast, by Type (2019-2030)
11.2.5.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.2.5.4. Market Revenue and Forecast, by Application (2019-2030)
11.2.6. Germany
11.2.6.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.2.6.2. Market Revenue and Forecast, by Type (2019-2030)
11.2.6.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.2.6.4. Market Revenue and Forecast, by Application (2019-2030)
11.2.7. France
11.2.7.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.2.7.2. Market Revenue and Forecast, by Type (2019-2030)
11.2.7.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.2.7.4. Market Revenue and Forecast, by Application (2019-2030)
11.2.8. Rest of Europe
11.2.8.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.2.8.2. Market Revenue and Forecast, by Type (2019-2030)
11.2.8.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.2.8.4. Market Revenue and Forecast, by Application (2019-2030)
11.3. APAC
11.3.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.3.2. Market Revenue and Forecast, by Type (2019-2030)
11.3.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.3.4. Market Revenue and Forecast, by Application (2019-2030)
11.3.5. India
11.3.5.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.3.5.2. Market Revenue and Forecast, by Type (2019-2030)
11.3.5.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.3.5.4. Market Revenue and Forecast, by Application (2019-2030)
11.3.6. China
11.3.6.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.3.6.2. Market Revenue and Forecast, by Type (2019-2030)
11.3.6.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.3.6.4. Market Revenue and Forecast, by Application (2019-2030)
11.3.7. Japan
11.3.7.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.3.7.2. Market Revenue and Forecast, by Type (2019-2030)
11.3.7.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.3.7.4. Market Revenue and Forecast, by Application (2019-2030)
11.3.8. Rest of APAC
11.3.8.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.3.8.2. Market Revenue and Forecast, by Type (2019-2030)
11.3.8.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.3.8.4. Market Revenue and Forecast, by Application (2019-2030)
11.4. MEA
11.4.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.4.2. Market Revenue and Forecast, by Type (2019-2030)
11.4.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.4.4. Market Revenue and Forecast, by Application (2019-2030)
11.4.5. GCC
11.4.5.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.4.5.2. Market Revenue and Forecast, by Type (2019-2030)
11.4.5.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.4.5.4. Market Revenue and Forecast, by Application (2019-2030)
11.4.6. North Africa
11.4.6.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.4.6.2. Market Revenue and Forecast, by Type (2019-2030)
11.4.6.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.4.6.4. Market Revenue and Forecast, by Application (2019-2030)
11.4.7. South Africa
11.4.7.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.4.7.2. Market Revenue and Forecast, by Type (2019-2030)
11.4.7.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.4.7.4. Market Revenue and Forecast, by Application (2019-2030)
11.4.8. Rest of MEA
11.4.8.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.4.8.2. Market Revenue and Forecast, by Type (2019-2030)
11.4.8.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.4.8.4. Market Revenue and Forecast, by Application (2019-2030)
11.5. Latin America
11.5.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.5.2. Market Revenue and Forecast, by Type (2019-2030)
11.5.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.5.4. Market Revenue and Forecast, by Application (2019-2030)
11.5.5. Brazil
11.5.5.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.5.5.2. Market Revenue and Forecast, by Type (2019-2030)
11.5.5.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.5.5.4. Market Revenue and Forecast, by Application (2019-2030)
11.5.6. Rest of LATAM
11.5.6.1. Market Revenue and Forecast, by Type of Manufacturer (2019-2030)
11.5.6.2. Market Revenue and Forecast, by Type (2019-2030)
11.5.6.3. Market Revenue and Forecast, by Type of Synthesis (2019-2030)
11.5.6.4. Market Revenue and Forecast, by Application (2019-2030)
Chapter 12. Company Profiles
12.1. Albemarle Corporation
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. AurobindoPharma
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. Dr. Reddy’s Laboratories Ltd.
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. AbbVieInc
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Teva Pharmaceutical Industries Ltd
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. Mylan N.V.
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. CiplaInc
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. BoehringerIngelheim International GmbH
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. Merck & Co., Inc
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. Sun Pharmaceutical Industries Ltd
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
12.11. Bristol-Myers Squibb Company
12.11.1. Company Overview
12.11.2. Product Offerings
12.11.3. Financial Performance
12.11.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
Buy This Premium Research Report Click Here@ https://www.precedenceresearch.com/checkout/1035
About Us
Precedence Research is a Canada/India based company and one of the leading providers of strategic market insights. We offer executive-level blueprints of markets and solutions beyond flagship surveys. Our repository covers consultation, syndicated market studies, and customized research reports. Through our services we aim at connecting an organization’s goal with lucrative prospects globally.
From gauging investment feasibility to uncovering hidden growth opportunities, our market studies cover in-depth analysis, which also is interspersed with relevant statistics. Recommendation are often enclosed within our reports with the sole intent of enabling organizations achieve mission-critical success.
Contact Us:
Precedence Research
Apt 1408 1785 Riverside Drive Ottawa, ON, K1G 3T7, Canada
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Website: https://www.precedenceresearch.com