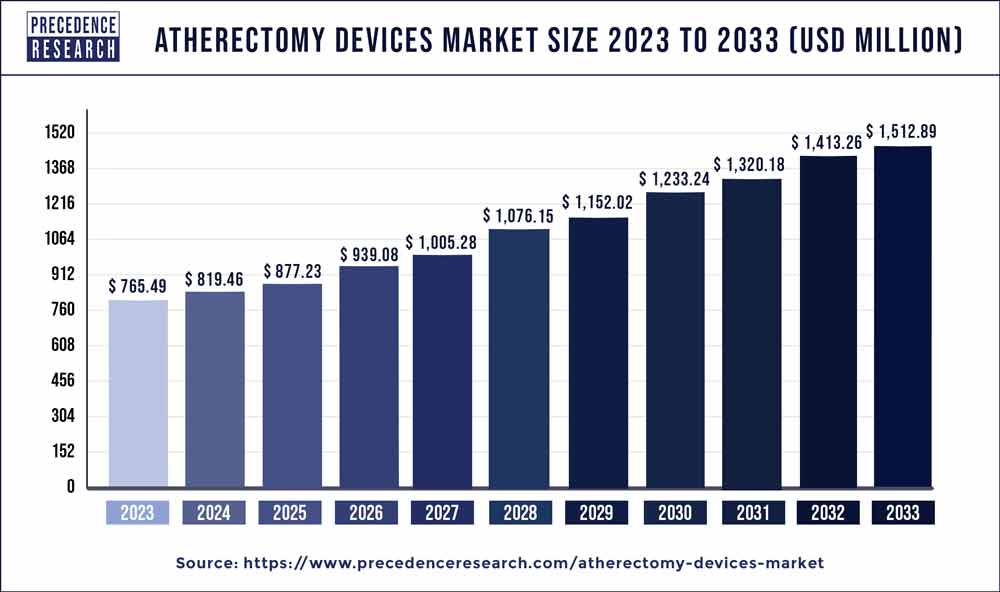
According to a recent research report titled “Atherectomy Devices Market (By Devices Type: Atherectomy Devices, Angioplasty Balloon Catheters, Stents (Bare-Metal, Drug-Eluting, Bioresorbable), Intravascular Ultrasound Catheters, Optical Coherence Tomography (OCT) Catheters, Drug-Coated Balloons, Embolic Protection Devices, Thrombectomy Devices, Aortic Stent Grafts, Endovascular Grafts, Laser Atherectomy Devices, and Others; By Application; By End user:) – Global Industry Analysis, Size, Share, Growth, Trends, Regional Outlook, and Forecast 2024-2033″ published by Precedence Research, the global atherectomy devices market size is projected to touch around USD 1,512.89 million by 2033 and growing at a CAGR of 7.05% over the forecast period 2024 to 2033. This comprehensive study examines various factors and their impact on the growth of the atherectomy devices market.
Key Points
- North America led the market with the biggest market share of 45% in 2023.
- Asia Pacific is projected to expand at the fastest rate during the forecast period of 2024-2033.
- By device type, the drug-coated balloons (DCBs) segment held the largest share of the market in 2023.
- By device type, the intravascular ultrasound (IVUS) catheter segment is expected to show the fastest growth.
- By application type, the peripheral artery disease segment held the dominating share of the market in 2023.
- By application type, the coronary artery disease segment represents another highly influential segment for the forecast period.
- By end-user, the cardiac catheterization labs segment held the dominating share of the market in 2023.
- By end-user, the interventional radiology departments segment is expected to witness a significant rate of expansion during the forecast period.
The report primarily focuses on the volume and value of the atherectomy devices market at the global, regional, and company levels. At the global level, the report analyzes historical data and future prospects to present an overview of the overall market size. Regionally, the study emphasizes key regions such as North America, Europe, the Middle East & Africa, Latin America, and others.
Furthermore, the research report provides specific segmentations based on regions (countries), companies, and all market segments. This analysis offers insights into the growth and revenue trends during the historical period of 2021 to 2033, as well as the projected period. By understanding these segments, it becomes possible to identify the significance of different factors that contribute to market growth.
Download a Free Copy of Our Latest Sample Report@ https://www.precedenceresearch.com/sample/3901
The research also highlights significant progressions in both organic and inorganic growth strategies within the global atherectomy devices market. Numerous companies are placing emphasis on new product launches, gaining product approvals, and implementing various business expansion tactics. Moreover, the report presents detailed profiles of firms operating in the atherectomy devices market, along with their respective market strategies. Additionally, the study concentrates on prominent industry participants, furnishing details such as company profiles, product offerings, financial updates, and noteworthy advancements.
Atherectomy Devices Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 7.05% |
| Global Market Size in 2023 | USD 765.49 Million |
| Global Market Size by 2033 | USD 1,512.89 Million |
| U.S. Market Size in 2023 | USD 241.13 Million |
| U.S. Market Size by 2033 | USD 476.56 Million |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Devices Type, By Application, and By End-user |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Also read: Green Cement Market Size to Rise USD 1,046.76 Million by 2033
Major Key Points Covered in the Report:
Executive Summary: It includes key trends of the electric vehicle fuel cell market related to products, applications, and other crucial factors. It also provides an analysis of the competitive landscape and CAGR and market size of the electric vehicle fuel cell market based on production and revenue.
Production and Consumption by Region: It covers all regional markets to which the research study relates. Prices and key players in addition to production and consumption in each regional market are discussed.
Key Players: Here, the report throws light on financial ratios, pricing structure, production cost, gross profit, sales volume, revenue, and gross margin of leading and prominent companies competing in the Electric vehicle fuel cell market.
Market Segments: This part of the report discusses product, application and other segments of the electric vehicle fuel cell market based on market share, CAGR, market size, and various other factors.
Research Methodology: This section discusses the research methodology and approach used to prepare the report. It covers data triangulation, market breakdown, market size estimation, and research design and/or programs.
Market Key Players
The report incorporates company profiles of key players in the market. These profiles encompass vital information such as product portfolio, key strategies, and a comprehensive SWOT analysis for each player. Additionally, the report presents a matrix illustrating the presence of each prominent player, enabling readers to gain actionable insights. This facilitates a thoughtful assessment of the market status and aids in predicting the level of competition in the atherectomy devices market.
Atherectomy Devices Market Companies
- Abbott Laboratories
- Boston Scientific Corporation
- BD
- Cardinal Health Inc.
- Koninklijke Philips NV
- Medtronic Plc
- Terumo Corporation
- Avinger
- Cardiovascular Systems
- Ra Medical Systems
Segments Covered in the Report
By Devices Type
- Atherectomy Devices
- Angioplasty Balloon Catheters
- Stents (Bare-Metal, Drug-Eluting, Bioresorbable)
- Intravascular Ultrasound (IVUS) Catheters
- Optical Coherence Tomography (OCT) Catheters
- Drug-Coated Balloons
- Embolic Protection Devices
- Thrombectomy Devices
- Aortic Stent Grafts
- Endovascular Grafts
- Laser Atherectomy Devices
- Orbital Atherectomy Systems
- Rotational Atherectomy Devices
- Directional Atherectomy Devices
- Chronic Total Occlusion (CTO) Devices
By Application
- Peripheral Artery Disease (PAD) Treatment Devices
- Coronary Artery Disease (CAD) Intervention Devices
- Carotid Artery Disease Intervention Devices
- Renal Artery Disease Intervention Devices
- Aortic Atherosclerosis Intervention Devices
By End-user
- Cardiac Catheterization Labs
- Interventional Radiology Departments
- Vascular Surgery Centers
- Cardiology Clinics
- Academic Research Institutions
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Table of Content
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Atherectomy Devices Market
5.1. COVID-19 Landscape: Atherectomy Devices Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Atherectomy Devices Market, By Devices Type
8.1. Atherectomy Devices Market Revenue and Volume, by Devices Type, 2024-2033
8.1.1 Atherectomy Devices
8.1.1.1. Market Revenue and Volume Forecast (2021-2033)
8.1.2. Angioplasty Balloon Catheters
8.1.2.1. Market Revenue and Volume Forecast (2021-2033)
8.1.3. Stents (Bare-Metal, Drug-Eluting, Bioresorbable)
8.1.3.1. Market Revenue and Volume Forecast (2021-2033)
8.1.4. Intravascular Ultrasound (IVUS) Catheters
8.1.4.1. Market Revenue and Volume Forecast (2021-2033)
8.1.5. Optical Coherence Tomography (OCT) Catheters
8.1.5.1. Market Revenue and Volume Forecast (2021-2033)
8.1.6. Drug-Coated Balloons
8.1.6.1. Market Revenue and Volume Forecast (2021-2033)
8.1.7. Embolic Protection Devices
8.1.7.1. Market Revenue and Volume Forecast (2021-2033)
8.1.8. Thrombectomy Devices
8.1.8.1. Market Revenue and Volume Forecast (2021-2033)
8.1.9. Aortic Stent Grafts
8.1.9.1. Market Revenue and Volume Forecast (2021-2033)
8.1.10. Endovascular Grafts
8.1.10.1. Market Revenue and Volume Forecast (2021-2033)
8.1.11. Laser Atherectomy Devices
8.1.11.1. Market Revenue and Volume Forecast (2021-2033)
8.1.12. Orbital Atherectomy Systems
8.1.12.1. Market Revenue and Volume Forecast (2021-2033)
8.1.13. Rotational Atherectomy Devices
8.1.13.1. Market Revenue and Volume Forecast (2021-2033)
8.1.14. Directional Atherectomy Devices
8.1.14.1. Market Revenue and Volume Forecast (2021-2033)
8.1.15. Chronic Total Occlusion (CTO) Devices
8.1.15.1. Market Revenue and Volume Forecast (2021-2033)
Chapter 9. Global Atherectomy Devices Market, By Application
9.1. Atherectomy Devices Market Revenue and Volume, by Application, 2024-2033
9.1.1. Peripheral Artery Disease (PAD) Treatment Devices
9.1.1.1. Market Revenue and Volume Forecast (2021-2033)
9.1.2. Coronary Artery Disease (CAD) Intervention Devices
9.1.2.1. Market Revenue and Volume Forecast (2021-2033)
9.1.3. Carotid Artery Disease Intervention Devices
9.1.3.1. Market Revenue and Volume Forecast (2021-2033)
9.1.4. Renal Artery Disease Intervention Devices
9.1.4.1. Market Revenue and Volume Forecast (2021-2033)
9.1.5. Aortic Atherosclerosis Intervention Devices
9.1.5.1. Market Revenue and Volume Forecast (2021-2033)
Chapter 10. Global Atherectomy Devices Market, By End-user
10.1. Atherectomy Devices Market Revenue and Volume, by End-user, 2024-2033
10.1.1. Cardiac Catheterization Labs
10.1.1.1. Market Revenue and Volume Forecast (2021-2033)
10.1.2. Interventional Radiology Departments
10.1.2.1. Market Revenue and Volume Forecast (2021-2033)
10.1.3. Vascular Surgery Centers
10.1.3.1. Market Revenue and Volume Forecast (2021-2033)
10.1.4. Cardiology Clinics
10.1.4.1. Market Revenue and Volume Forecast (2021-2033)
10.1.5. Academic Research Institutions
10.1.5.1. Market Revenue and Volume Forecast (2021-2033)
Chapter 11. Global Atherectomy Devices Market, Regional Estimates and Trend Forecast
11.1. North America
11.1.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.1.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.1.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.1.4. U.S.
11.1.4.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.1.4.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.1.4.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.1.5. Rest of North America
11.1.5.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.1.5.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.1.5.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.2. Europe
11.2.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.2.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.2.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.2.4. UK
11.2.4.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.2.4.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.2.4.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.2.5. Germany
11.2.5.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.2.5.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.2.5.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.2.6. France
11.2.6.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.2.6.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.2.6.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.2.7. Rest of Europe
11.2.7.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.2.7.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.2.7.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.3. APAC
11.3.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.3.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.3.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.3.4. India
11.3.4.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.3.4.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.3.4.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.3.5. China
11.3.5.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.3.5.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.3.5.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.3.6. Japan
11.3.6.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.3.6.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.3.6.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.3.7. Rest of APAC
11.3.7.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.3.7.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.3.7.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.4. MEA
11.4.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.4.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.4.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.4.4. GCC
11.4.4.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.4.4.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.4.4.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.4.5. North Africa
11.4.5.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.4.5.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.4.5.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.4.6. South Africa
11.4.6.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.4.6.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.4.6.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.4.7. Rest of MEA
11.4.7.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.4.7.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.4.7.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.5. Latin America
11.5.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.5.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.5.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.5.4. Brazil
11.5.4.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.5.4.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.5.4.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
11.5.5. Rest of LATAM
11.5.5.1. Market Revenue and Volume Forecast, by Devices Type (2021-2033)
11.5.5.2. Market Revenue and Volume Forecast, by Application (2021-2033)
11.5.5.3. Market Revenue and Volume Forecast, by End-user (2021-2033)
Chapter 12. Company Profiles
12.1. Abbott Laboratories
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. Boston Scientific Corporation
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. BD
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Cardinal Health Inc.
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Koninklijke Philips NV
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. Medtronic Plc
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. Terumo Corporation
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. Avinger
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. Cardiovascular Systems
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. Ra Medical Systems
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
Why should you invest in this report?
This report presents a compelling investment opportunity for those interested in the global atherectomy devices market. It serves as an extensive and informative guide, offering clear insights into this niche market. By delving into the report, you will gain a comprehensive understanding of the various major application areas for atherectomy devices. Furthermore, it provides crucial information about the key regions worldwide that are expected to experience substantial growth within the forecast period of 2024-2033. Armed with this knowledge, you can strategically plan your market entry approaches.
Moreover, this report offers a deep analysis of the competitive landscape, equipping you with valuable insights into the level of competition prevalent in this highly competitive market. If you are already an established player, it will enable you to assess the strategies employed by your competitors, allowing you to stay ahead as market leaders. For newcomers entering this market, the extensive data provided in this report is invaluable, providing a solid foundation for informed decision-making.
Some of the key questions answered in this report:
- What is the size of the overall Atherectomy devices market and its segments?
- What are the key segments and sub-segments in the market?
- What are the key drivers, restraints, opportunities and challenges of the Atherectomy devices market and how they are expected to impact the market?
- What are the attractive investment opportunities within the Atherectomy devices market?
- What is the Atherectomy devices market size at the regional and country-level?
- Who are the key market players and their key competitors?
- What are the strategies for growth adopted by the key players in Atherectomy devices market?
- What are the recent trends in Atherectomy devices market? (M&A, partnerships, new product developments, expansions)?
- What are the challenges to the Atherectomy devices market growth?
- What are the key market trends impacting the growth of Atherectomy devices market?
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.pharma-geek.com