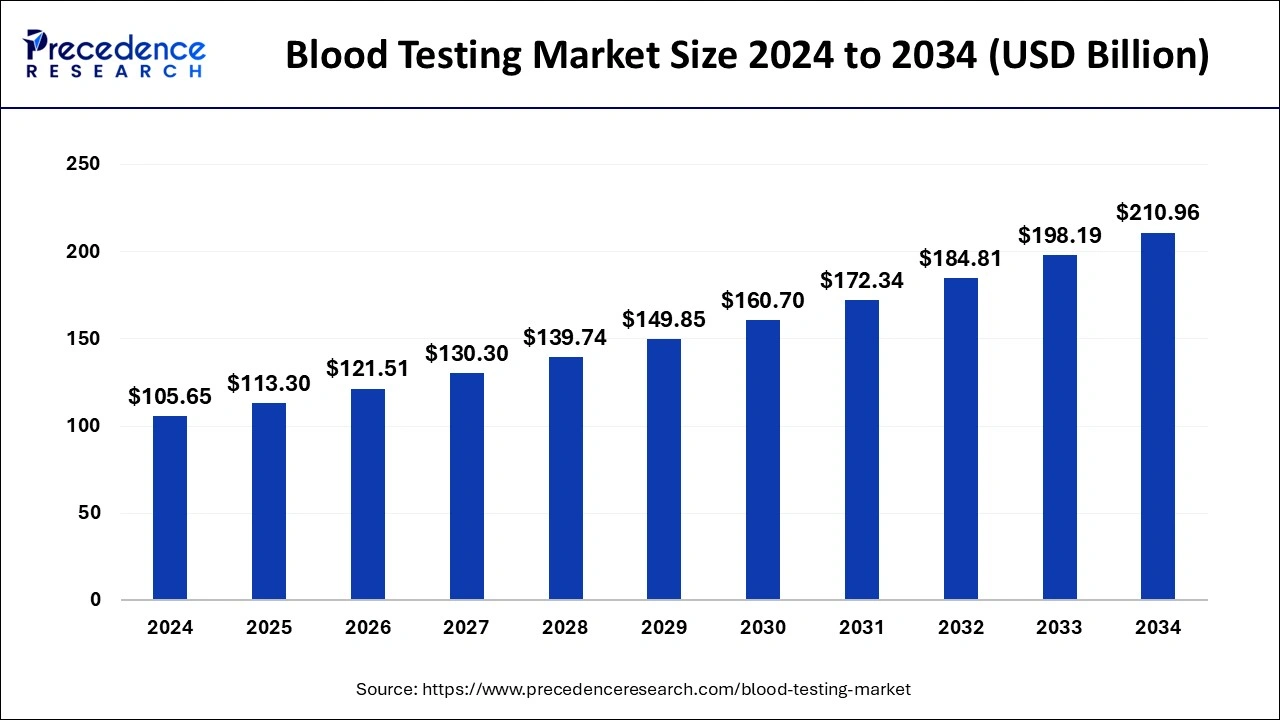
The global blood testing market size was valued at USD 98.52 billion in 2023 and is expected to reach around USD 198.19 billion by 2033, expanding at a CAGR of 7.24% from 2024 to 2033.
Key Points
- The North America blood testing market size is exhibited at USD 43.35 billion in 2023 and is expected to attain around USD 88.19 billion by 2033, poised to grow at a CAGR of 7.36% between 2024 and 2033.
- North America led the market with the largest revenue share of 44% in 2023.
- Asia-Pacific is observed to experience the fastest rate of growth during the forecast period.
- By test type, the glucose segment has contributed more than 18% of revenue share in 2023.
The global blood testing market encompasses a wide array of diagnostic tests performed on blood samples to evaluate various health conditions. These tests are crucial in clinical settings for disease detection, monitoring of treatment effectiveness, and overall health assessment. Key factors driving the growth of this market include increasing prevalence of chronic diseases, advancements in diagnostic technologies, rising awareness about early disease detection, and the aging population worldwide.
Get a Sample: https://www.precedenceresearch.com/sample/4459
Growth Factors
The blood testing market is experiencing significant growth due to several factors. Firstly, the increasing incidence of chronic diseases such as diabetes, cardiovascular diseases, and cancer necessitates regular blood tests for early diagnosis and monitoring. Secondly, technological advancements in laboratory techniques, including the development of automated and high-throughput testing systems, have improved the accuracy and efficiency of blood tests. Thirdly, rising healthcare expenditure and investments in healthcare infrastructure across emerging economies are expanding access to diagnostic services, thereby boosting market growth.
Region Insights
Geographically, North America and Europe dominate the blood testing market due to well-established healthcare infrastructure, high healthcare expenditure, and early adoption of advanced diagnostic technologies. In North America, the United States holds a significant share owing to the presence of major market players, extensive research activities, and favorable reimbursement policies. Asia-Pacific is expected to witness rapid growth driven by increasing healthcare spending, improving healthcare facilities, and growing awareness about preventive healthcare measures.
Blood Testing Market Scope
| Report Coverage | Details |
| Market Size in 2023 | USD 98.52 Billion |
| Market Size in 2024 | USD 105.65 Billion |
| Market Size by 2033 | USD 198.19 Billion |
| Market Growth Rate from 2024 to 2033 | CAGR of 7.24% |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Blood Testing Market Dynamics
Drivers
Several drivers propel the blood testing market forward. Advances in personalized medicine and the increasing demand for point-of-care testing (POCT) devices are enhancing market growth. Additionally, the shift towards preventive healthcare and the integration of artificial intelligence (AI) and machine learning in diagnostic procedures are streamlining testing processes and improving diagnostic accuracy. Moreover, initiatives by governments and healthcare organizations to promote routine health check-ups and early disease detection are encouraging the adoption of blood tests among the population.
Opportunities
The blood testing market presents numerous opportunities for growth and innovation. Expansion in telemedicine services and the development of portable diagnostic devices are opening new avenues for market players. Moreover, increasing research and development activities in biomarker discovery and genomic testing are expected to revolutionize personalized medicine and drive market expansion. Furthermore, strategic collaborations between healthcare providers, diagnostic laboratories, and technology developers can lead to the introduction of novel diagnostic solutions and expand market reach in underserved regions.
Challenges
Despite the promising growth prospects, the blood testing market faces several challenges. These include stringent regulatory requirements for diagnostic tests, particularly in developed regions, which can delay market entry for new products. Moreover, concerns regarding data privacy and security in healthcare remain a significant barrier to the adoption of digital health solutions. Additionally, the variability in reimbursement policies across different regions poses challenges for market penetration, particularly in emerging economies where healthcare infrastructure and reimbursement mechanisms are evolving.
Read Also: Artificial Intelligence (AI) in Epidemiology Market Size, Report By 2033
Blood Testing Market Companies
- Abbott
- F. Hoffmann-La Roche AG
- Bio-Rad Laboratories, Inc.
- BioMerieux SA
- Quest Diagnostics
- Biomerica, Inc.
- Becton, Dickinson and Company
- Siemens Healthineers
- Danaher Corporation
- Trinity Biotech Plc
- Sinocare Inc
- Becton Dickson & Company
Recent Developments
- In April 2024, Quest Diagnostics launched a p-tau217 blood biomarker test. This test is done to diagnose patients suffering from Alzheimer’s Disease.
- In March 2024, Labcorp launched the pTau217 blood biomarker test. This test will help to identify the prevalence of Alzheimer’s disease among the patients.
- In December 2023, Savara launched a new blood test for treating patients suffering from lung disorders. The blood test is named ‘aPAP ClearPath’ that can help doctors to treat patients suffering from rare lung diseases called ‘autoimmune pulmonary alveolar proteinosis (aPAP).
- In August 2023, C2N Diagnostics launched the PrecivityAD2 blood test. This blood test is done to assess patients suffering from mild Alzheimer’s disease.
- In July 2023, Quanterix Corporation launched a new biomarker blood test for diagnosing Alzheimer’s disease. This test is named ‘LucentAD’, and it helps in diagnosing patients experiencing cognitive symptoms of Alzheimer’s disease.
- In March 2023, Abbott announced that the U.S. Food and Drug Administration (FDA) had approved laboratory traumatic brain injury (TBI) blood tests in the U.S.. This new blood test method will help to reduce the waiting times in hospitals and replace CT scans for examining mild traumatic brain injuries.
Segments Covered in the Report
By Test Type
- Glucose Testing
- A1C Testing
- Direct LDL Testing
- Lipid Panel Testing
- Prostate-specific Antigen Testing
- COVID-19 Testing
- BUN Testing
- Vitamin D Testing
- Thyroid-stimulating Hormone (TSH)
- Serum Nicotine/Cotinine
- High-sensitivity CRP Testing
- Testosterone Testing
- ALT Testing
- Cortisol Testing
- Creatinine Testing
- AST Testing
- Other Blood Tests
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/