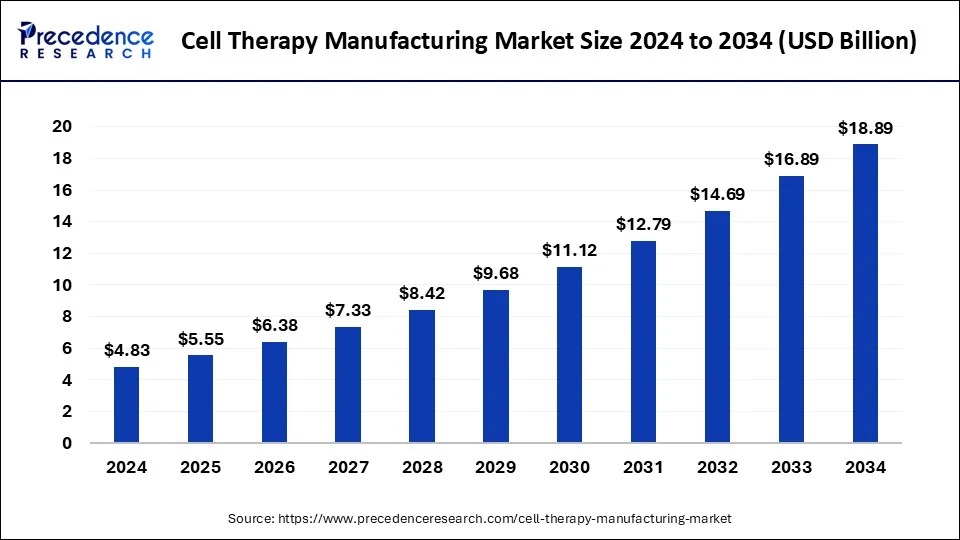
The global cell therapy manufacturing market size is estimated to rake around USD 16.89 billion by 2033, growing at a CAGR of 14.93% from 2024 to 2033.
Key Points
- The North America cell therapy manufacturing market size accounted for USD 1.85 billion in 2023 and is expected to attain around USD 7.43 billion by 2033.
- North America led the market with the major revenue share of 44% in 2023.
- Asia Pacific is expected to witness the fastest growth during the forecast period.
- By therapy type, the autologous cell therapy segment has held the biggest revenue share of 59% in 2023.
- By therapy type, the allogenic cell therapy segment is projected to be the fastest-growing segment over the forecast period.
- By technology type, the somatic cell technology segment held the largest share of the market in 2023.
- By technology type, the 3D technology segment is expected to grow at the fastest rate during the projected period.
- By source, the IPSC (induced pluripotent stem cell) segment dominated the market in 2023.
- By source, the bone marrow segment is the second largest segment in the global market.
- By application, the oncology segment has contributed the largest revenue share of 35% in 2023.
- By application, the neurological segment is projected to show fastest growth during the forecast period.
The cell therapy manufacturing market is a rapidly evolving sector within the broader field of biopharmaceuticals. It involves the production and processing of cells for therapeutic applications, including cell-based immunotherapies, regenerative medicine, and stem cell therapies. The market encompasses various stages of manufacturing, from cell sourcing and isolation to cell expansion, genetic modification (if applicable), and final product formulation.
Get a Sample: https://www.precedenceresearch.com/sample/4283
Growth Factors
Several key factors are driving the growth of the cell therapy manufacturing market. One significant factor is the increasing prevalence of chronic diseases and cancer, which has fueled the demand for innovative cell-based therapies. Additionally, advancements in biotechnology and genetic engineering have led to the development of more sophisticated cell manufacturing techniques, making cell therapies more viable and scalable for commercial production. Moreover, growing investments by pharmaceutical companies and government initiatives to support research in cell therapy are contributing to market expansion.
Region Insights
The cell therapy manufacturing market exhibits regional variations influenced by factors such as regulatory frameworks, infrastructure, and market demand. North America, particularly the United States, dominates the market due to a strong biotechnology sector, favorable regulatory environment, and substantial investments in research and development. Europe is also a significant region, with countries like Germany and the UK playing key roles in advancing cell therapy manufacturing. Meanwhile, the Asia-Pacific region is emerging as a promising market, driven by increasing healthcare expenditure, growing biopharmaceutical industry, and rising awareness about personalized medicine.
Cell Therapy Manufacturing Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 14.93% |
| Cell Therapy Manufacturing Market Size in 2023 | USD 4.20 Billion |
| Cell Therapy Manufacturing Market Size in 2024 | USD 4.83 Billion |
| Cell Therapy Manufacturing Market Size by 2033 | USD 16.89 Billion |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Therapy Type, By Technology Type, By Source, and By Application |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Cell Therapy Manufacturing Market Dynamics
Drivers
Several drivers are propelling the growth of the cell therapy manufacturing market. Technological advancements in cell culture techniques, such as automation and closed-system processing, are improving efficiency and reducing manufacturing costs. Moreover, the shift towards personalized medicine and the potential of cell therapies to address unmet medical needs are driving investments and collaborations in this space. Additionally, regulatory agencies’ increasing acceptance of cell therapies as viable treatment options is boosting confidence among stakeholders and facilitating market growth.
Opportunities
The cell therapy manufacturing market presents diverse opportunities for stakeholders across the value chain. Expansion into emerging markets, particularly in Asia-Pacific and Latin America, offers new avenues for market penetration and growth. Furthermore, collaborations between academic institutions, biotechnology companies, and healthcare providers can accelerate innovation and streamline manufacturing processes. Opportunities also exist in developing novel technologies for cell characterization, cryopreservation, and scalability to meet the growing demand for cell therapies.
Challenges
Despite significant growth prospects, the cell therapy manufacturing market faces several challenges. One of the primary challenges is the complexity of manufacturing processes, particularly for personalized therapies requiring patient-specific cells. Scalability and cost-effectiveness remain critical hurdles in commercializing cell therapies. Additionally, ensuring product consistency, maintaining quality standards, and navigating evolving regulatory landscapes pose challenges to market participants. Addressing these challenges requires continued investment in research, process optimization, and regulatory strategies.
Read Also: Brazil Industrial Absorbent Market Size, Growth, Report by 2033
Cell Therapy Manufacturing Market Recent Developments
- In March 2024, Cellars announced the completion of the first Cell Shuttle, an automated, cGMP-compliant cell therapy manufacturing platform to support the global demand for cell therapies while reducing the cost of manufacturing and process failure rates.
- In March 2023, Cell One Partners and the Center for Breakthrough Medicines (CBM) entered into a strategic collaboration aimed at accelerating the development and commercialization of cell and gene therapies. This collaboration brings together the expertise and resources of both organizations to drive innovation and advance the field of regenerative medicine.
- In September 2022, the Cell Therapy Manufacturing Center (CTMC) and Ori Bio collaborated to expedite the process development, commercialization, and clinical integration of cell therapies. This partnership aimed to enhance the advancement of cell-based treatments and bring them to patients more efficiently.
Cell Therapy Manufacturing Market Companies
- Merck KGaA
- Thermo Fisher Scientific
- Catalent, Inc
- Bio-Techne
- Cytiva
- Lonza
- The Discovery Labs
- Novartis AG
- Bristol-Myers Squibb Company
- Gilead Sciences, Inc.
Segments Covered in the Report
By Therapy Type
- Allogenic Cell Therapy
- Autologous Cell Therapy
By Technology Type
- Somatic Cell Technology
- Cell Immortalization Technology
- Viral Vector Technology
- Genome Editing Technology
- Cell Plasticity Technology
- 3D Technology
By Source
- IPSC (Induced Pluripotent Stem Cell)
- Bone Marrow
- Umbilical Cord
- Adipose Tissues
- Neural Stem
By Application
- Musculoskeletal
- Cardiovascular
- Gastrointestinal
- Neurological
- Oncology
- Dermatology
- Others
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/