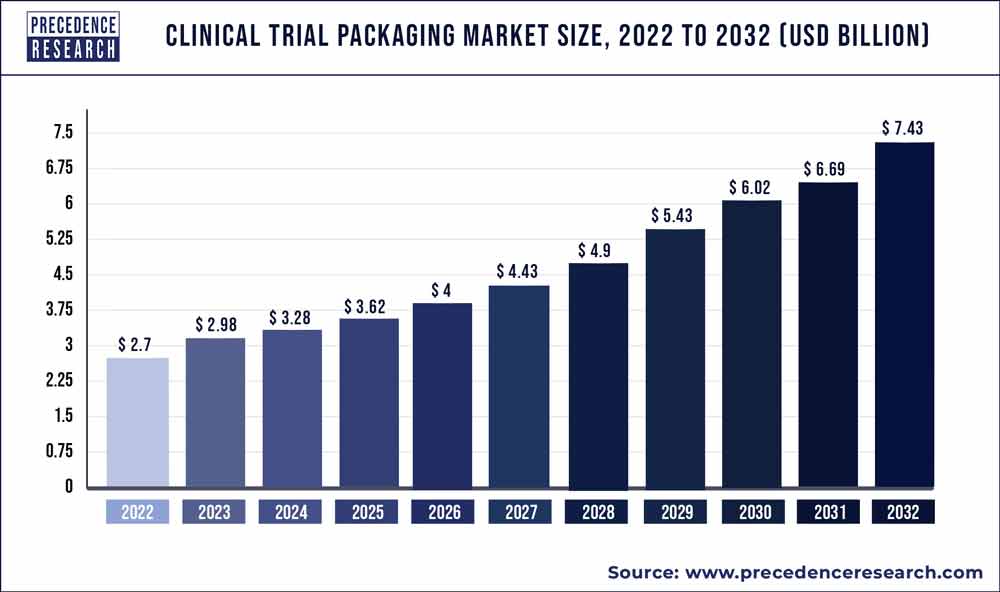
According to Precedence Research, during the forecast period of 2022 to 2030, the global clinical trial packaging market is estimated to develop at a compound annual growth rate (CAGR) of 11.19%. The global clinical trial packaging market was valued at USD 2.89 billion in 2021, and it is predicted to exceed USD 7.51 billion by 2030. The study investigates several elements and their consequences on the growth of the clinical trial packaging market.
Download Free Sample Copy with TOC@ https://www.precedenceresearch.com/sample/2067
This report focuses on clinical trial packaging market volume and value at the global level, regional level and company level. From a global perspective, this report represents overall clinical trial packaging market size by analyzing historical data and future prospect. Regionally, this report focuses on several key regions: North America, Europe, Middle East & Africa, Latin America, etc.
The research report includes specific segments by region (country), by company, by all segments. This study provides information about the growth and revenue during the historic and forecasted period of 2017 to 2030. Understanding the segments helps in identifying the importance of different factors that aid the market growth.
Report Scope of the Clinical Trial Packaging Market
| Report Coverage | Details |
| Market Size in 2022 | USD 3.21 Billion |
| Market Size by 2030 | USD 7.51 Billion |
| Growth Rate from 2022 to 2030 | CAGR of 11.19% |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segments Covered | Clinical Trial Type, Packaging Type, Material, End User and Geography |
In-Depth Analysis on Competitive Landscape
The report sheds light on leading manufacturers of clinical trial packaging, along with their detailed profiles. Essential and up-to-date data related to market performers who are principally engaged in the production of clinical trial packaging has been brought with the help of a detailed dashboard view. Market share analysis and comparison of prominent players provided in the report permits report readers to take preemptive steps in advancing their businesses.
Company profiles have been included in the report, which include essentials such as product portfolio, key strategies, along with all-inclusive SWOT analysis on each player. Company presence is mapped and presented through a matrix for all the prominent players, thus providing readers with actionable insights, which helps in thoughtfully presenting market status and predicting the competition level in the clinical trial packaging market.
Some of the prominent players in the clinical trial packaging market include:
- Bilcare
- Fisher Clinical Services
- WuXi AppTec
- PCI Pharma Services
- Almac Group
- PharMaterials
- PAREXEL
- Schreiner MediPharm
- Sharp Packaging
- The Coghlan Group
- Rubicon
- Westrock
- Xerimis
- Catalent
- Piramal Pharma Solutions
- Corden Pharma
- DMB Consultancy
- Körber Medipak Systems
- Sentry BioPharma
- NextPharma
- Mawdsleys
Ask Here For More Customization Study@ https://www.precedenceresearch.com/customization/2067
Segments Covered in the Report
By Clinical Trial Type
- Therapeutic and Prevention
- Vaccines
- Drug Discovery and Development
- Therapeutic Devices
- Biosimilars
- Therapeutic Assays
- Therapeutic Procedures
- Diagnostics
- Diagnostic Assay
- Diagnostic Devices
By Packaging Type
- Syringes
- Vials & Ampoules
- Blisters
- Cold Forming
- Thermoforming
- Tubes
- Bottles
- Bags & Pouches
- Sachets
- Kits or Packs
- Others
By Material
- Plastic
- PVC
- PE
- HDPE
- LDPE
- PP
- Others
- Glass
- Metal
- Paper
- Corrugated Fiber
By End User
- Research Laboratories
- Clinical Research Organizations
- Drug Manufacturing Facilities
Regional Segmentation
- Asia-Pacific [China, Southeast Asia, India, Japan, Korea, Western Asia]
- Europe [Germany, UK, France, Italy, Russia, Spain, Netherlands, Turkey, Switzerland]
- North America [United States, Canada, Mexico]
- South America [Brazil, Argentina, Columbia, Chile, Peru]
- Middle East & Africa [GCC, North Africa, South Africa]
Some of the important ones are:
- What can be the best investment choices for venturing into new product and service lines?
- What value propositions should businesses aim at while making new research and development funding?
- Which regulations will be most helpful for stakeholders to boost their supply chain network?
- Which regions might see the demand maturing in certain segments in near future?
- What are the some of the best cost optimization strategies with vendors that some well-entrenched players have gained success with?
- Which are the key perspectives that the C-suite are leveraging to move businesses to new growth trajectory?
- Which government regulations might challenge the status of key regional markets?
- How will the emerging political and economic scenario affect opportunities in key growth areas?
- What are some of the value-grab opportunities in various segments?
- What will be the barrier to entry for new players in the market?
Table of Contents
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Clinical Trial Packaging Market
5.1. COVID-19 Landscape: Clinical Trial Packaging Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Clinical Trial Packaging Market, By Clinical Trial Type
8.1. Clinical Trial Packaging Market, by Clinical Trial Type, 2022-2030
8.1.1. Therapeutic and Prevention
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Diagnostics
8.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Clinical Trial Packaging Market, By Packaging Type
9.1. Clinical Trial Packaging Market, by Packaging Type e, 2022-2030
9.1.1. Syringes
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Vials & Ampoules
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. Blisters
9.1.3.1. Market Revenue and Forecast (2017-2030)
9.1.4. Tubes
9.1.4.1. Market Revenue and Forecast (2017-2030)
9.1.5. Bottles
9.1.5.1. Market Revenue and Forecast (2017-2030)
9.1.6. Bags & Pouches
9.1.6.1. Market Revenue and Forecast (2017-2030)
9.1.7. Sachets
9.1.7.1. Market Revenue and Forecast (2017-2030)
9.1.8. Kits or Packs
9.1.8.1. Market Revenue and Forecast (2017-2030)
9.1.9. Others
9.1.9.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Clinical Trial Packaging Market, By Material
10.1. Clinical Trial Packaging Market, by Material, 2022-2030
10.1.1. Plastic
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Glass
10.1.2.1. Market Revenue and Forecast (2017-2030)
10.1.3. Metal
10.1.3.1. Market Revenue and Forecast (2017-2030)
10.1.4. Paper
10.1.4.1. Market Revenue and Forecast (2017-2030)
10.1.5. Corrugated Fiber
10.1.5.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global Clinical Trial Packaging Market, By End User
11.1. Clinical Trial Packaging Market, by End User, 2022-2030
11.1.1. Research Laboratories
11.1.1.1. Market Revenue and Forecast (2017-2030)
11.1.2. Clinical Research Organizations
11.1.2.1. Market Revenue and Forecast (2017-2030)
11.1.3. Drug Manufacturing Facilities
11.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 12. Global Clinical Trial Packaging Market, Regional Estimates and Trend Forecast
12.1. North America
12.1.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.1.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.1.3. Market Revenue and Forecast, by Material (2017-2030)
12.1.4. Market Revenue and Forecast, by End User (2017-2030)
12.1.5. U.S.
12.1.5.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.1.5.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.1.5.3. Market Revenue and Forecast, by Material (2017-2030)
12.1.5.4. Market Revenue and Forecast, by End User (2017-2030)
12.1.6. Rest of North America
12.1.6.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.1.6.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.1.6.3. Market Revenue and Forecast, by Material (2017-2030)
12.1.6.4. Market Revenue and Forecast, by End User (2017-2030)
12.2. Europe
12.2.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.2.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.2.3. Market Revenue and Forecast, by Material (2017-2030)
12.2.4. Market Revenue and Forecast, by End User (2017-2030)
12.2.5. UK
12.2.5.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.2.5.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.2.5.3. Market Revenue and Forecast, by Material (2017-2030)
12.2.5.4. Market Revenue and Forecast, by End User (2017-2030)
12.2.6. Germany
12.2.6.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.2.6.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.2.6.3. Market Revenue and Forecast, by Material (2017-2030)
12.2.6.4. Market Revenue and Forecast, by End User (2017-2030)
12.2.7. France
12.2.7.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.2.7.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.2.7.3. Market Revenue and Forecast, by Material (2017-2030)
12.2.7.4. Market Revenue and Forecast, by End User (2017-2030)
12.2.8. Rest of Europe
12.2.8.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.2.8.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.2.8.3. Market Revenue and Forecast, by Material (2017-2030)
12.2.8.4. Market Revenue and Forecast, by End User (2017-2030)
12.3. APAC
12.3.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.3.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.3.3. Market Revenue and Forecast, by Material (2017-2030)
12.3.4. Market Revenue and Forecast, by End User (2017-2030)
12.3.5. India
12.3.5.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.3.5.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.3.5.3. Market Revenue and Forecast, by Material (2017-2030)
12.3.5.4. Market Revenue and Forecast, by End User (2017-2030)
12.3.6. China
12.3.6.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.3.6.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.3.6.3. Market Revenue and Forecast, by Material (2017-2030)
12.3.6.4. Market Revenue and Forecast, by End User (2017-2030)
12.3.7. Japan
12.3.7.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.3.7.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.3.7.3. Market Revenue and Forecast, by Material (2017-2030)
12.3.7.4. Market Revenue and Forecast, by End User (2017-2030)
12.3.8. Rest of APAC
12.3.8.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.3.8.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.3.8.3. Market Revenue and Forecast, by Material (2017-2030)
12.3.8.4. Market Revenue and Forecast, by End User (2017-2030)
12.4. MEA
12.4.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.4.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.4.3. Market Revenue and Forecast, by Material (2017-2030)
12.4.4. Market Revenue and Forecast, by End User (2017-2030)
12.4.5. GCC
12.4.5.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.4.5.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.4.5.3. Market Revenue and Forecast, by Material (2017-2030)
12.4.5.4. Market Revenue and Forecast, by End User (2017-2030)
12.4.6. North Africa
12.4.6.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.4.6.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.4.6.3. Market Revenue and Forecast, by Material (2017-2030)
12.4.6.4. Market Revenue and Forecast, by End User (2017-2030)
12.4.7. South Africa
12.4.7.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.4.7.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.4.7.3. Market Revenue and Forecast, by Material (2017-2030)
12.4.7.4. Market Revenue and Forecast, by End User (2017-2030)
12.4.8. Rest of MEA
12.4.8.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.4.8.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.4.8.3. Market Revenue and Forecast, by Material (2017-2030)
12.4.8.4. Market Revenue and Forecast, by End User (2017-2030)
12.5. Latin America
12.5.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.5.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.5.3. Market Revenue and Forecast, by Material (2017-2030)
12.5.4. Market Revenue and Forecast, by End User (2017-2030)
12.5.5. Brazil
12.5.5.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.5.5.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.5.5.3. Market Revenue and Forecast, by Material (2017-2030)
12.5.5.4. Market Revenue and Forecast, by End User (2017-2030)
12.5.6. Rest of LATAM
12.5.6.1. Market Revenue and Forecast, by Clinical Trial Type (2017-2030)
12.5.6.2. Market Revenue and Forecast, by Packaging Type (2017-2030)
12.5.6.3. Market Revenue and Forecast, by Material (2017-2030)
12.5.6.4. Market Revenue and Forecast, by End User (2017-2030)
Chapter 13. Company Profiles
13.1. Bilcare
13.1.1. Company Overview
13.1.2. Product Offerings
13.1.3. Financial Performance
13.1.4. Recent Initiatives
13.2. Fisher Clinical Services
13.2.1. Company Overview
13.2.2. Product Offerings
13.2.3. Financial Performance
13.2.4. Recent Initiatives
13.3. WuXi AppTec
13.3.1. Company Overview
13.3.2. Product Offerings
13.3.3. Financial Performance
13.3.4. Recent Initiatives
13.4. PCI Pharma Services
13.4.1. Company Overview
13.4.2. Product Offerings
13.4.3. Financial Performance
13.4.4. Recent Initiatives
13.5. Almac Group
13.5.1. Company Overview
13.5.2. Product Offerings
13.5.3. Financial Performance
13.5.4. Recent Initiatives
13.6. PharMaterials
13.6.1. Company Overview
13.6.2. Product Offerings
13.6.3. Financial Performance
13.6.4. Recent Initiatives
13.7. PAREXEL
13.7.1. Company Overview
13.7.2. Product Offerings
13.7.3. Financial Performance
13.7.4. Recent Initiatives
13.8. Schreiner MediPharm
13.8.1. Company Overview
13.8.2. Product Offerings
13.8.3. Financial Performance
13.8.4. Recent Initiatives
13.9. Sharp Packaging
13.9.1. Company Overview
13.9.2. Product Offerings
13.9.3. Financial Performance
13.9.4. Recent Initiatives
13.10. The Coghlan Group
13.10.1. Company Overview
13.10.2. Product Offerings
13.10.3. Financial Performance
13.10.4. Recent Initiatives
Chapter 14. Research Methodology
14.1. Primary Research
14.2. Secondary Research
14.3. Assumptions
Chapter 15. Appendix
15.1. About Us
15.2. Glossary of Terms
Buy This Premium Research Report Click Here@ https://www.precedenceresearch.com/checkout/2067
About Us
Precedence Research is a Canada/India based company and one of the leading providers of strategic market insights. We offer executive-level blueprints of markets and solutions beyond flagship surveys. Our repository covers consultation, syndicated market studies, and customized research reports. Through our services we aim at connecting an organization’s goal with lucrative prospects globally.
From gauging investment feasibility to uncovering hidden growth opportunities, our market studies cover in-depth analysis, which also is interspersed with relevant statistics. Recommendation are often enclosed within our reports with the sole intent of enabling organizations achieve mission-critical success.
Contact Us:
Precedence Research
Apt 1408 1785 Riverside Drive Ottawa, ON, K1G 3T7, Canada
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Website: https://www.precedenceresearch.com