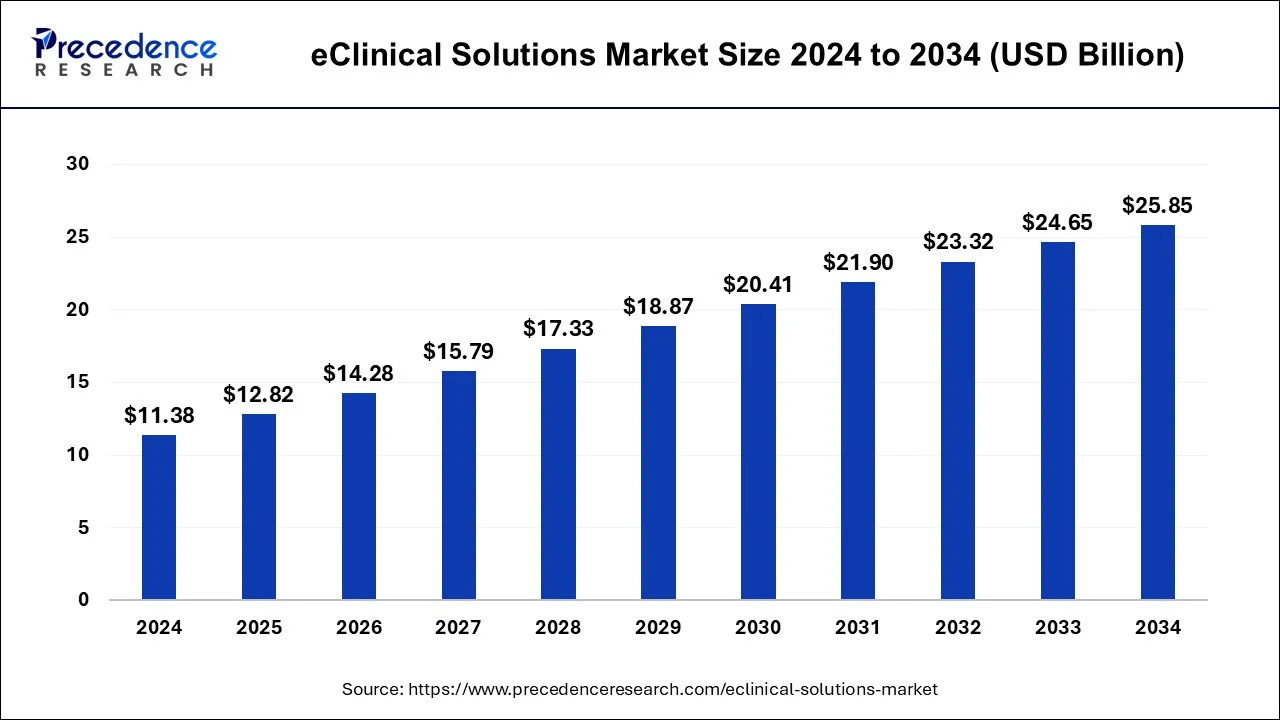
The eClinical solutions market, valued at $11.38B in 2024, is set to reach $25.85B by 2034, growing at a steady CAGR of 8.10%
eClinical Solutions Market Key Takeaways
- North America led the eClinical solutions market in 2024, holding the largest market share of 47.93%.
- Web-hosted delivery mode dominated the market, accounting for over 50.49% of total revenue in 2024.
- Phase III development phase held the largest revenue share, contributing over 43.71% to the eClinical solutions market in 2024.
- Contract Research Organizations (CROs) segment led the market, capturing a 39.38% revenue share in 2024.
The eClinical solutions market is experiencing significant growth, driven by advancements in digital technologies for clinical trials. In 2024, North America held the largest market share at 47.93%, while the web-hosted delivery mode dominated with over 50.49% of revenue. The phase III development phase accounted for the highest revenue share at 43.71%, and the Contract Research Organizations (CROs) segment led the market with a 39.38% share. With increasing adoption of AI, cloud computing, and data analytics, the market is expected to expand further in the coming years.
Sample Link: https://www.precedenceresearch.com/sample/1092
Key Drivers
Opportunities
- Expansion of Decentralized Trials – Growing demand for remote and hybrid clinical trials is creating opportunities for innovative eClinical solutions.
- Advancements in AI & Machine Learning – AI-driven automation and predictive analytics enhance clinical trial efficiency and data accuracy.
- Rising Investments in Healthcare IT – Increasing funding in digital healthcare infrastructure supports market expansion.
- Growing Adoption in Emerging Markets – Developing regions are witnessing increased adoption of eClinical solutions due to evolving healthcare regulations.
- Integration of Blockchain Technology – Blockchain can improve data security, transparency, and regulatory compliance in clinical trials.
Challenges
- Data Security & Privacy Concerns – Managing and protecting sensitive clinical trial data remains a critical challenge.
- Regulatory Complexity – Varying global regulations can make compliance difficult for eClinical solution providers.
- High Implementation Costs – The initial investment in eClinical technologies can be expensive, limiting adoption by smaller organizations.
- Resistance to Digital Adoption – Some organizations still rely on traditional clinical trial methods, slowing the transition to digital solutions.
- Integration Issues with Legacy Systems – Compatibility challenges with existing clinical trial infrastructure can hinder seamless implementation.
Regional Insights
North America dominates the eClinical solutions market, holding the largest share due to the presence of leading pharmaceutical companies, advanced healthcare infrastructure, and strong regulatory frameworks supporting digital adoption. Europe follows closely, driven by increasing clinical trials, government funding, and a growing focus on personalized medicine.
The Asia-Pacific region is experiencing rapid growth, fueled by rising investments in healthcare technology, expanding clinical research activities, and supportive government initiatives in countries like China and India. Latin America is also witnessing steady adoption, with improvements in healthcare infrastructure and regulatory advancements. The Middle East and Africa region, while still emerging, is gradually adopting eClinical solutions due to increasing clinical research collaborations and growing investments in digital healthcare.
Don’t Miss Out: Laboratory Equipment Market
Market Key Players
- Medidata Solution
- DATATRAK
- ERT Clinical
- CRF Health
- eClinicalWorks
- OmniComm Systems
- IBM Watson Health
- eClinical Solutions
Recent News
The eClinical solutions market is experiencing significant growth, driven by technological advancements and increased adoption in clinical trials. In February 2025, a strategic industry report projected the market to reach USD 22.7 billion by 2030, with a CAGR of 12.2% from 2024 to 2030. Similarly, another analysis estimated the market size at USD 9.2 billion in 2023, expecting it to grow at a CAGR of 14.1% from 2024 to 2030.
In December 2024, eClinical Solutions was recognized as the highest leader in Everest Group’s Life Sciences Clinical Data and Analytics Platforms PEAK Matrix Assessment 2024. This accolade underscores the company’s commitment to innovation and excellence in the field. The market’s expansion is further supported by the rising complexity of clinical trials and the growing adoption of digital solutions to enhance data management and trial efficiency. As the industry continues to evolve, eClinical solutions are poised to play a pivotal role in transforming clinical research and development processes.
Market Segmentation
By Solution
- Randomization and Trial Supply Management
- Clinical Data Management System
- Clinical Trial Management System
- Electronic Clinical Outcome Assessment
- Electronic Trial Master Files
- Electronic Data Capture
- Others
By Development Phase
- Phase IV
- Phase III
- Phase II
- Phase I
By Delivery Mode
- Licensed Enterprise
- Web-hosted
- Cloud-based
By End-Use
- CROs
- Hospitals
- Academic Institutes
- Medical Device Manufacturers
- Pharma & Biotech Organizations