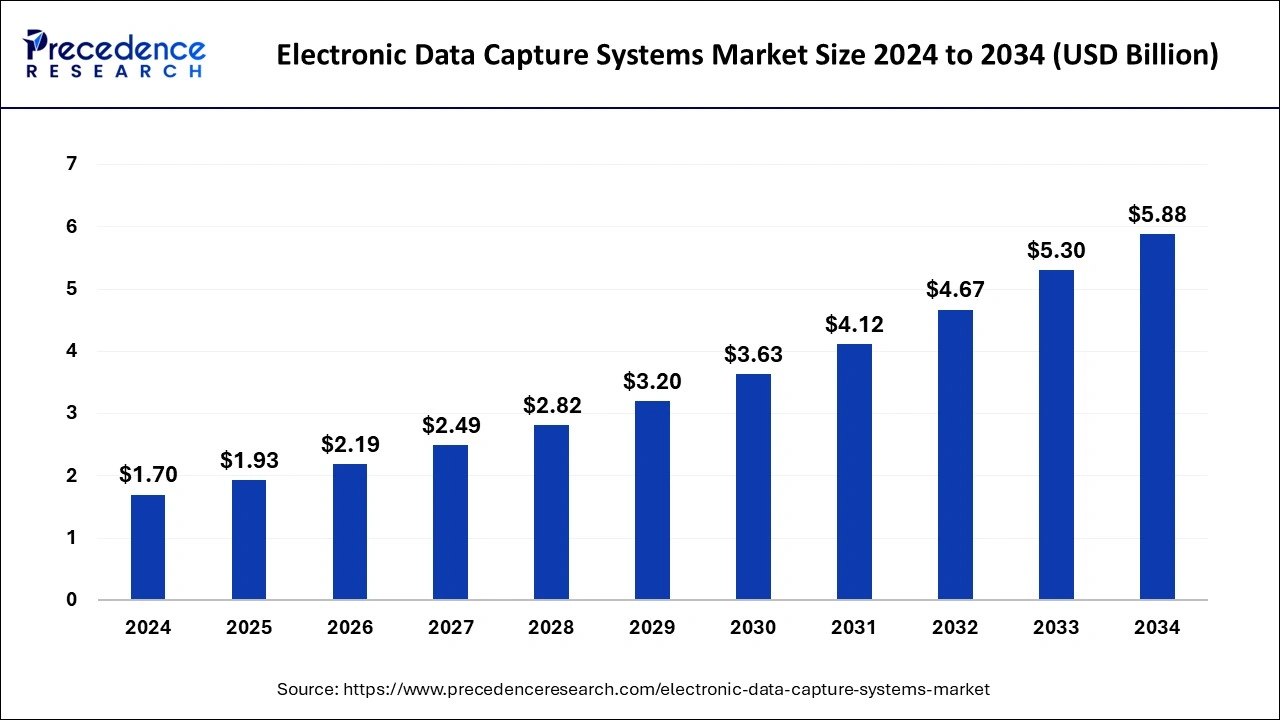
The global electronic data capture systems market size is anticipated to attain around USD 5.30 billion by 2033, growing at a CAGR of 13.45% from 2024 to 2033
Key Points
- The North America electronic data capture systems market size reached USD 770 million in 2023 and is projected to hit around USD 2,700 million by 2033.
- North America dominated the electronic data capture systems market in 2023 with a market share of 51%.
- By the development phase, the Phase III segment has accounted largest market share of 50.8% in 2023.
- By end user, the CROs segment held the dominating share of around 36% in 2023.
- By end user, the hospitals or healthcare providers segment is showcasing a lucrative growth during the forecast period.
- By delivery mode segment, the web and cloud-based systems segment dominated the market and held the largest share in 2023.
- Based on component, the highest share was captured by the services segment of the electronic data capture systems market and thus dominated the market on a global scale.
- The software segment of component is expected to witness a significant growth rate during the forecast period.
The Electronic Data Capture (EDC) Systems market is a rapidly growing sector within the healthcare industry. EDC systems are digital platforms used to collect, store, and manage clinical data in clinical trials and research studies. They offer significant advantages over traditional paper-based data collection methods, including increased efficiency, accuracy, and accessibility of data. The market’s growth is driven by the increasing need for efficient data management in clinical trials, advancements in healthcare technology, and the rise in the number of clinical research projects worldwide.
Get a Sample: https://www.precedenceresearch.com/sample/4090
Growth Factors
Several factors are contributing to the growth of the Electronic Data Capture Systems market:
- Rising Demand for Efficient Data Management: As clinical trials become more complex and data-intensive, the need for efficient and reliable data management solutions increases. EDC systems offer streamlined data collection, organization, and analysis, which is crucial for the success of clinical trials.
- Advancements in Healthcare Technology: The healthcare industry is experiencing rapid advancements in technology, including cloud computing, AI, and machine learning. These technologies are being integrated into EDC systems, enhancing their capabilities and making them more attractive to healthcare providers and researchers.
- Increased Focus on Patient Safety and Regulatory Compliance: Regulatory authorities such as the FDA and EMA are placing greater emphasis on data integrity and patient safety in clinical trials. EDC systems help researchers adhere to these regulations by providing accurate and verifiable data.
- Rise in Clinical Research Projects: The number of clinical trials and research projects has increased significantly worldwide, driven by the demand for new treatments and therapies. This trend is expected to continue, driving the demand for EDC systems.
- Adoption of Decentralized Clinical Trials (DCTs): The adoption of decentralized clinical trials, which involve the use of digital technologies to conduct trials remotely, is boosting the demand for EDC systems. These systems enable researchers to collect data from participants in real-time, regardless of their location.
Region Insights
The EDC systems market is geographically segmented into North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa. Each region presents unique opportunities and challenges:
- North America: North America dominates the EDC systems market due to its advanced healthcare infrastructure, high adoption of technology, and significant investment in clinical research. The presence of key market players and regulatory support further drive the market’s growth in this region.
- Europe: Europe is another major market for EDC systems, with strong growth driven by the region’s focus on patient safety and regulatory compliance. The European Medicines Agency (EMA) plays a significant role in shaping the market’s trajectory.
- Asia-Pacific: The Asia-Pacific region is expected to experience substantial growth in the coming years. This growth is attributed to the rising number of clinical trials, increasing healthcare expenditure, and growing awareness of the benefits of EDC systems. Countries like China and India are emerging as key players in the region.
- Latin America: Latin America is also witnessing an increase in clinical research activities, leading to the adoption of EDC systems. However, the region faces challenges such as regulatory hurdles and a lack of skilled professionals.
- Middle East and Africa: While the Middle East and Africa represent smaller markets for EDC systems, they hold potential for growth due to the increasing investment in healthcare infrastructure and the rising number of clinical trials in these regions.
Electronic Data Capture Systems Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 13.45% |
| Global Market Size in 2023 | USD 1.50 Billion |
| Global Market Size in 2024 | USD 1.70 Billion |
| Global Market Size by 2033 | USD 5.30 Billion |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Delivery Mode, By Component, By Development Phase, and By End-user |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Electronic Data Capture Systems Market Dynamics
Drivers
The key drivers of the EDC systems market include:
- Efficiency and Accuracy: EDC systems improve the efficiency and accuracy of data collection and management in clinical trials, reducing errors and saving time.
- Cost-Effectiveness: By automating data collection and management, EDC systems help researchers save costs associated with manual data entry and processing.
- Regulatory Compliance: EDC systems assist researchers in adhering to regulatory standards by providing accurate and verifiable data.
- Growing Adoption of Technology: The increasing adoption of cloud-based solutions, AI, and machine learning in EDC systems enhances their capabilities and appeal.
- Demand for Real-Time Data: Real-time data collection and analysis enable researchers to make informed decisions quickly, improving the efficiency of clinical trials.
Challenges
Despite the growth prospects, the EDC systems market faces several challenges:
- Data Security Concerns: Ensuring the security and confidentiality of sensitive clinical data is a significant challenge for EDC system providers.
- Interoperability Issues: EDC systems often need to integrate with other healthcare systems and devices, which can present interoperability challenges.
- High Implementation Costs: The initial cost of implementing EDC systems can be high, particularly for smaller research organizations.
- Lack of Standardization: The lack of standardization in EDC systems can lead to compatibility issues and hinder the adoption of these systems.
- Resistance to Change: Some researchers and organizations may be resistant to adopting new technologies due to concerns about learning curves and disruptions to existing workflows.
Opportunities
The EDC systems market presents several opportunities for growth:
- Integration with Other Technologies: Integrating EDC systems with other technologies such as AI, machine learning, and blockchain can enhance their capabilities and offer new solutions.
- Expansion into Emerging Markets: Emerging markets such as Asia-Pacific and Latin America offer significant growth opportunities due to increasing healthcare investment and clinical research activities.
- Development of Customized Solutions: Developing EDC systems tailored to specific research needs can attract a broader range of customers and improve adoption rates.
- Focus on Patient-Centric Solutions: Designing EDC systems with a focus on patient-centricity can improve data quality and participant engagement.
- Collaborative Partnerships: Forming partnerships with other healthcare technology companies and research institutions can lead to new innovations and market expansion.
Read Also: Battery Binders Market Size to Reach USD 8.20 Billion by 2033
Recent Developments
- In March 2024, Veeva Systems Inc. VEEV recently announced that Veeva Vault EDC has powered more than 1,000 study starts. Per management, this reflects increased adoption of Vault EDC, which is enabling companies to establish two contract research organizations (CROs) standardizing Vault EDC.
- In September 2023- Medidata a Dassault Systeme’s company, was rated the pharmaceutical industry’s preferred provider of electronic data capture (EDC) solutions in a new report by Industry Standard Research. This report also identified Medidata’s EDC system as the No. 1 most recently used EDC system across the industry.
Electronic Data Capture Systems Market Companies
- Calyx
- Castor
- Open Clinica, LLC
- IBM
- IQVIA Inc.
- Medidata Solutions, Inc.
- Oracle
- DATATRAK International, Inc.
- Clario
- Veeva Systems
Segments Covered in the Report
By Delivery Mode
- On-premises
- Web & Cloud-based
By Component
- Software
- Services
By Development Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By End-user
- Hospitals/Healthcare Providers
- CROs
- Pharmaceutical and Biotechnology Firms
- Medical Device Firms
- Others
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/