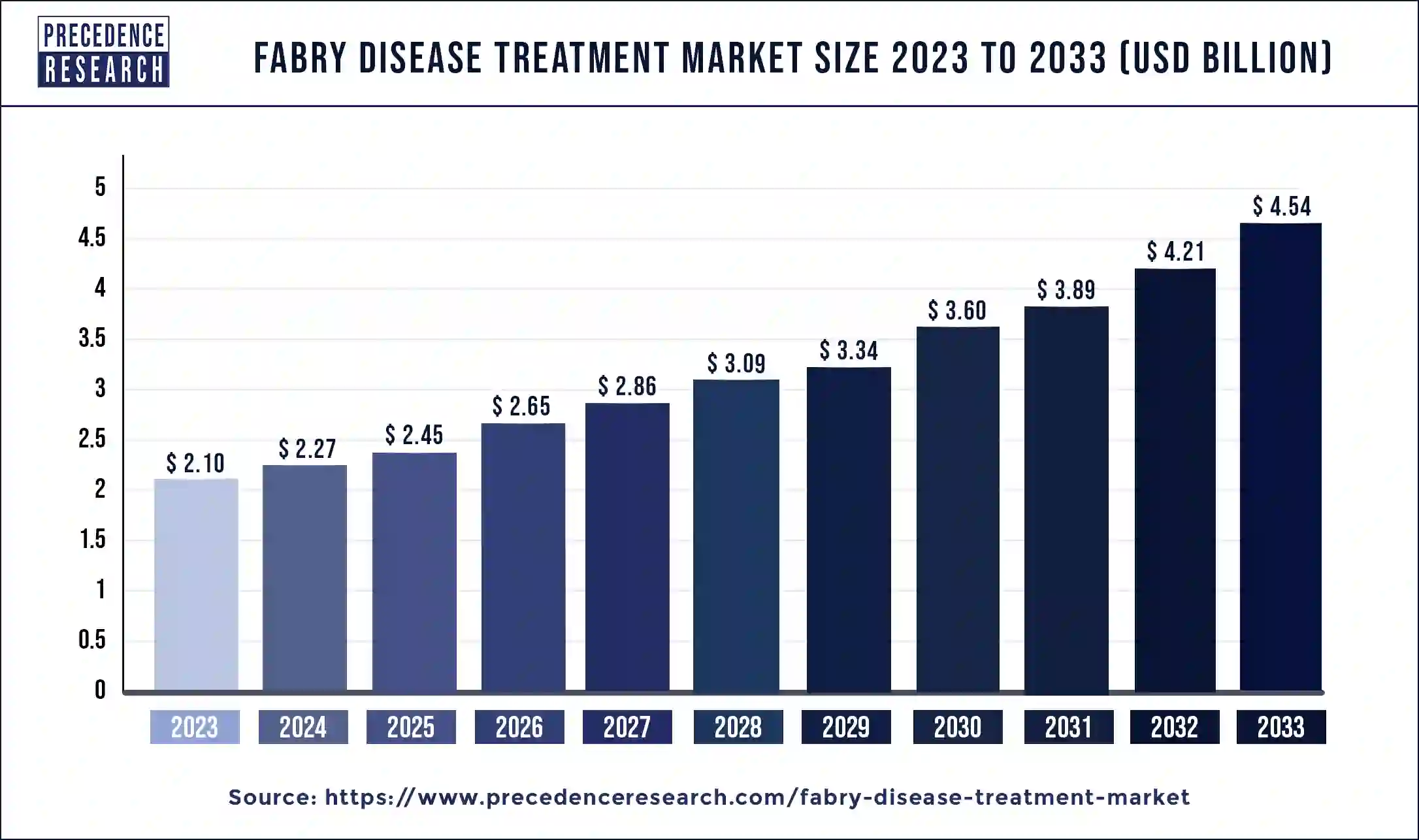
The global fabry disease treatment market size was estimated at USD 2.10 billion in 2023 and is predicted to be worth around USD 4.54 billion by 2033, growing at a CAGR of 8.02% from 2024 to 2033.
Key Points
- The North America fabry disease treatment market size accounted for USD 710 million in 2023 and is expected to attain around USD 1,570 million by 2033, poised to grow at a CAGR of 8.25% between 2024 and 2033.
- North America dominated the market in 2023.
- Asia Pacific is expected to host the fastest-growing market over the forecast period.
- By treatment, the enzyme replacement therapy segment dominated the market in 2023.
- By treatment, the substrate reduction therapy segment grows at a rapid pace in the market over the forecast period.
Fabry disease is a rare genetic disorder that results from the buildup of a specific type of fat in the body’s cells, leading to a range of symptoms such as pain, kidney failure, heart issues, and more. The Fabry disease treatment market focuses on therapies and medications designed to manage and alleviate these symptoms. With advances in medical research and biotechnology, the market is evolving to offer more effective and personalized treatment options, including enzyme replacement therapy (ERT), chaperone therapy, substrate reduction therapy (SRT), and gene therapy.
Get a Sample: https://www.precedenceresearch.com/sample/4404
Table of Contents
ToggleGrowth Factors
Several factors are driving the growth of the Fabry disease treatment market. First, increasing awareness and diagnosis of rare diseases have led to earlier and more accurate identification of Fabry disease, thereby increasing the demand for treatments. Advances in genetic research and biotechnology have facilitated the development of innovative therapies, particularly gene therapy, which holds promise for more effective long-term solutions. Additionally, the growing number of clinical trials and the approval of new drugs by regulatory bodies have expanded the treatment options available to patients.
Region Insights
The Fabry disease treatment market is segmented into various regions, including North America, Europe, Asia-Pacific, Latin America, and the Middle East and Africa. North America holds a significant share due to high healthcare expenditure, strong research infrastructure, and the presence of major pharmaceutical companies. Europe follows closely, benefiting from a well-established healthcare system and ongoing research initiatives. The Asia-Pacific region is expected to witness substantial growth due to increasing healthcare awareness, rising healthcare expenditures, and improving diagnostic capabilities. Emerging markets in Latin America and the Middle East and Africa are also anticipated to show growth as awareness and access to healthcare improve.
Fabry Disease Treatment Market Scope
| Report Coverage | Details |
| Fabry Disease Treatment Market Size in 2023 | USD 2.10 Billion |
| Fabry Disease Treatment Market Size in 2024 | USD 2.27 Billion |
| Fabry Disease Treatment Market Size by 2033 | USD 4.54 Billion |
| Fabry Disease Treatment Market Growth Rate | CAGR of 8.02% from 2024 to 2033 |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Fabry Disease Treatment Market Dynamics
Drivers
Key drivers of the Fabry disease treatment market include advancements in medical research, increased funding for rare disease research, and the rising prevalence of genetic disorders. The approval of novel therapies and the expansion of existing treatment protocols have significantly improved patient outcomes, fostering market growth. Additionally, supportive government policies and initiatives aimed at rare disease treatment are providing a conducive environment for market expansion.
Opportunities
The Fabry disease treatment market presents several opportunities for growth and development. The ongoing research and development of gene therapy offer the potential for curative treatments, which could revolutionize the management of Fabry disease. Personalized medicine, leveraging genetic and biomarker information, is another area with substantial growth potential, allowing for more tailored and effective treatment strategies. Furthermore, partnerships and collaborations between pharmaceutical companies, research institutions, and healthcare providers can accelerate the development and commercialization of innovative therapies.
Challenges
Despite the positive outlook, the Fabry disease treatment market faces several challenges. The high cost of treatment, particularly for advanced therapies like gene therapy, can limit accessibility for many patients. Limited awareness and understanding of the disease among both healthcare professionals and the general public can lead to delays in diagnosis and treatment. Additionally, the rarity of the disease makes it difficult to conduct large-scale clinical trials, which can hinder the development and approval of new treatments. Addressing these challenges requires concerted efforts from stakeholders across the healthcare spectrum.
Read Also: Embolic Protection Devices Market Size, Share, Report by 2033
Fabry Disease Treatment Market Recent Developments
- In May 2023, Chiesi Global Rare Diseases and Protalix BioTherapeutics, Inc. received FDA approval for Elfabrio (pegunigalsidase alfa-ix) in the United States for the treatment of adult patients with Fabry disease. Elfabrio is supplied as a preservative-free solution in a single-dose vial.
- In May 2023, Sangamo Therapeutics, Inc., a genomic medicine company, received Fast Track Designation from the FDA for isaralgagene civaparvovec, or ST-920, a wholly owned gene therapy product candidate for the treatment of Fabry disease. ST-920 is currently being evaluated in the Phase 1/2 STAAR study, with a total of 20 patients diagnosed to date.
- In May 2023, Europe allowed Chiesi Farmaceutici and Protalix BioTherapeutics to market their product PRX-102 (pegunigalsidase alfa) in the European territory as a treatment for Fabry disease.
- In September 2022, the agency granted the Orphan Drug Designation (ODD) to AL01211, produced by AceLink Therapeutics, for treating Fabry disease. It is a glucosylceramide synthase (GCS) inhibitor and has shown high potency. The treatment was claimed as a much-needed orally consumed medicine as compared to other available treatments.
Fabry Disease Treatment Market Companies
- JCR Pharmaceuticals
- Sanofi Genzyme
- Green Cross Corporation
- Chiesi Group
- Regenxbio Inc.
- Idorsia Pharmaceuticals
- Protalix BioTherapeutics
- Amicus Therapeutics
Segments Covered in the Report
By Treatment
- Enzyme Replacement Therapy (ERT)
- Chaperone Treatment
- Substrate Reduction Therapy (SRT)
- Others
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/