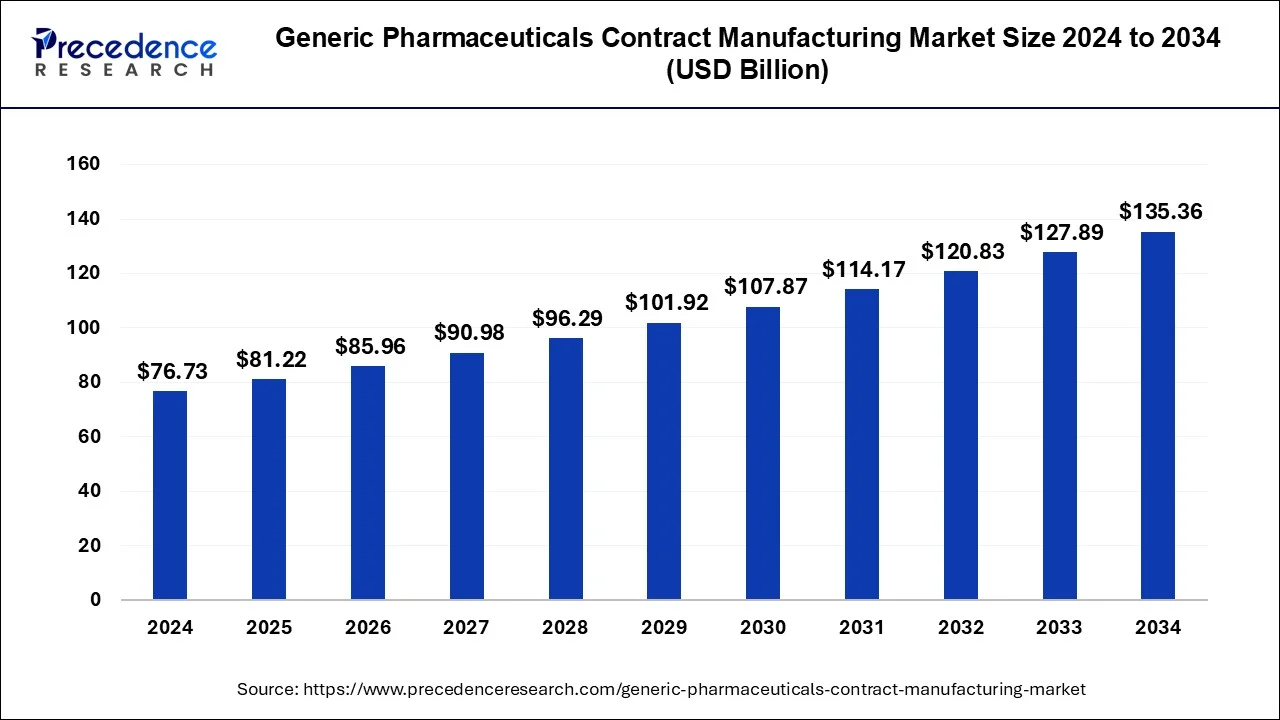
The global generic pharmaceuticals contract manufacturing market size was valued at USD 72.50 billion in 2023 and is predicted to be worth around USD 135.36 billion by 2034 with a CAGR of 5.84% from 2024 to 2034.
The generic pharmaceuticals contract manufacturing market involves the outsourcing of manufacturing processes by pharmaceutical companies to contract manufacturing organizations (CMOs). These CMOs specialize in producing generic drugs under license or as per the specifications provided by the client companies. This outsourcing model allows pharmaceutical companies to focus on their core competencies such as research and development (R&D), marketing, and distribution, while leveraging the manufacturing expertise and infrastructure of CMOs.
The market encompasses a wide range of services including formulation development, API (Active Pharmaceutical Ingredient) sourcing, manufacturing, packaging, and regulatory compliance. CMOs typically operate across various dosage forms including tablets, capsules, injectables, and others, catering to diverse therapeutic areas such as cardiovascular, central nervous system, oncology, and more.
Get a Sample: https://www.precedenceresearch.com/sample/4675
Generic Pharmaceuticals Contract Manufacturing Market Key Points
- North America is estimated to be the fastest-growing during the forecast period of 2024-2033.
- By drug type, the branded generics segment has contributed more than 63% of revenue share in 2023.
- By drug type, the unbranded generics segment is significantly growing during the forecast period.
- By product, the API product segment has held a major revenue share of 58% in 2023.
- By route of administration, the oral segment has captured the largest revenue share of 62% in 2023.
- By route of administration, the parenteral segment is anticipated to be the fastest-growing during the forecast period.
- By application, the oncology segment has generated the biggest revenue share of 23% in 2023.
- By application, the immunology segment is expected to be the fastest-growing during the forecast period.
Regional Insights
North America dominates the global generic pharmaceuticals contract manufacturing market, driven by the presence of several large pharmaceutical companies outsourcing manufacturing to CMOs. The region benefits from a well-established regulatory framework, advanced manufacturing technologies, and high demand for generic drugs due to cost containment efforts by healthcare providers.
Europe is another significant market for generic pharmaceuticals contract manufacturing, characterized by a robust pharmaceutical industry and stringent regulatory standards. The region’s market growth is supported by increasing investments in healthcare infrastructure and rising demand for affordable medicines. The Asia-Pacific region is experiencing rapid growth in the generic pharmaceuticals contract manufacturing market, fueled by factors such as low labor costs, large skilled workforce, favorable government initiatives, and increasing investments by pharmaceutical companies in emerging markets like India and China. These countries are emerging as key hubs for contract manufacturing due to their manufacturing capabilities and cost advantages.
Generic Pharmaceuticals Contract Manufacturing Market Trends
- Technological Advancements: The generic pharmaceuticals contract manufacturing market is witnessing advancements in manufacturing technologies such as continuous manufacturing, which offer benefits like reduced production time, improved product quality, and cost savings. This trend is driven by the need for efficiency and scalability in manufacturing operations.
- Biologics and Biosimilars: While traditionally focused on small molecule generics, there is a growing trend towards contract manufacturing of biologics and biosimilars. This segment requires specialized capabilities and infrastructure, presenting new growth opportunities for CMOs capable of handling complex biopharmaceutical processes.
- Quality and Compliance: There is an increasing emphasis on quality assurance and regulatory compliance in contract manufacturing. CMOs are investing in quality management systems, compliance with Good Manufacturing Practices (GMP), and adherence to stringent regulatory requirements across different markets to ensure product safety and efficacy.
- Vertical Integration: Some CMOs are adopting a strategy of vertical integration by expanding their service offerings to include API manufacturing, formulation development, and packaging services. This allows them to offer comprehensive solutions to pharmaceutical clients and enhance their competitiveness in the market.
- Shift Towards Emerging Markets: Pharmaceutical companies and CMOs are increasingly exploring opportunities in emerging markets for contract manufacturing. These markets offer attractive cost advantages, growing healthcare infrastructure, and a large patient pool, driving demand for generic medicines.
Generic Pharmaceuticals Contract Manufacturing Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 135.36 Billion |
| Market Size in 2023 | USD 72.50 Billion |
| Market Size in 2024 | USD 76.73 Billion |
| Market Growth Rate from 2024 to 2034 | CAGR of 5.84% |
| Largest Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2024 to 2034 |
| Segments Covered | Drug, Product, Route of Administration, Application, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Generic Pharmaceuticals Contract Manufacturing Market Dynamics
Drivers
- Cost Containment Efforts: Healthcare providers, governments, and insurers worldwide are under pressure to contain healthcare costs. Generic drugs, which are typically more affordable than branded counterparts, play a crucial role in achieving cost savings, thereby driving demand for generic pharmaceuticals and contract manufacturing services.
- Patent Expirations: The expiration of patents for blockbuster drugs is a significant driver for the generic pharmaceuticals market. Pharmaceutical companies seek to capitalize on the market opportunity presented by patent expiry by outsourcing manufacturing to CMOs capable of producing generic versions.
- Increasing Generic Drug Penetration: The global demand for generic drugs is on the rise due to factors such as aging populations, increasing prevalence of chronic diseases, and healthcare reforms promoting generic drug use. This trend boosts the demand for contract manufacturing services to meet the growing need for affordable medicines.
- Flexibility and Scalability: Contract manufacturing offers pharmaceutical companies flexibility in production capacity and scalability in response to fluctuating market demands. CMOs equipped with flexible manufacturing capabilities enable clients to quickly ramp up or scale down production volumes as needed, reducing time-to-market for new products.
Opportunities
- Emerging Markets Expansion: Expanding into emerging markets presents significant growth opportunities for CMOs. These markets offer large patient populations, increasing healthcare expenditure, and a favorable regulatory environment conducive to pharmaceutical manufacturing and outsourcing.
- Biopharmaceuticals and Biosimilars: The shift towards biologics and biosimilars presents lucrative opportunities for CMOs with expertise in biopharmaceutical manufacturing. The market for biosimilars is expected to grow as patents for biologic drugs expire, creating a demand for contract manufacturing services.
- Technological Innovations: Investments in advanced manufacturing technologies such as continuous manufacturing, 3D printing, and personalized medicine present opportunities for CMOs to differentiate their service offerings and cater to evolving client needs.
- Strategic Partnerships and Collaborations: Forming strategic partnerships with pharmaceutical companies, academic institutions, and research organizations can enable CMOs to access new technologies, expand their geographic presence, and enhance their service capabilities.
Challenges
- Regulatory Compliance: Meeting stringent regulatory requirements across different markets can be challenging for CMOs involved in global contract manufacturing. Variations in regulatory standards, documentation requirements, and inspections necessitate robust quality systems and compliance measures.
- Price Competition: Intense price competition in the generic pharmaceuticals market puts pressure on CMOs to offer competitive pricing while maintaining profitability. Price erosion, driven by multiple suppliers and low-cost manufacturing regions, poses a challenge to profitability margins.
- Intellectual Property Concerns: Intellectual property (IP) protection and confidentiality agreements are critical considerations in contract manufacturing agreements. CMOs must ensure strict adherence to IP rights and confidentiality provisions to mitigate risks related to data security and infringement issues.
- Supply Chain Complexity: Managing a complex global supply chain involving raw materials sourcing, logistics, and distribution is a logistical challenge for CMOs. Disruptions in the supply chain, including raw material shortages, transportation delays, and geopolitical uncertainties, can impact manufacturing timelines and product availability.
- Quality Control and Assurance: Maintaining consistent product quality and ensuring batch-to-batch consistency are paramount in pharmaceutical manufacturing. CMOs must invest in robust quality control systems, analytical testing capabilities, and process validation to meet customer expectations and regulatory standards.
Read Also: Facial Recognition Market Size to Reach USD 32.53 Bn by 2034
Generic Pharmaceuticals Contract Manufacturing Market Companies
- Metrics Contract Services
- Curia Global, Inc.
- Pfizer Centre One
- Syngene International Ltd.
- Acme Generics Pvt Ltd.
- Catalent, Inc.
- Alcami Corp., Inc.
- Cambrex Corp.
- Aurobindo Pharma
- Siegfried Holding AG
- Recipharm AB
- Jubilant Generics Ltd.
- Metrics Contract Services
Recent Developments
- In July 2023, Breyna Inhalation Aerosol, the first generic version of AstraZeneca’s Symbicort with an ANDA (abbreviated new drug application), was approved by the U.S. FDA (Food & Drug Administration) and launched by a global health company Viatris Inc. and Kindeva Drug Delivery L.P. for people with chronic obstructive pulmonary disease and asthma.
- In November 2023, an antibiotic manufacturing facility in Kundl, Austria was opened by a generic pharmaceuticals company based in Switzerland, Sandoz Group AG. In Europe, to strengthen the future of antibiotics manufacturing Sandoz invested $160.4m.
- In February 2024, in the United States, 5-6 new products in each quarter were planned to be launched by Alembic Pharmaceuticals, a Vadodara-based generics drugmaker.
Segments Covered in the Report
By Drug
- Branded Generics
- Unbranded Generics
By Product
- API
- Drug Product
By Route of Administration
- Oral
- Parenteral
- Topical
- Others
By Application
- Oncology
- Immunology
- Antidiabetic
- Neurology
- Anticoagulants
- Cardiovascular
- Respiratory
- Pain
- HIV antivirals
- Others
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/