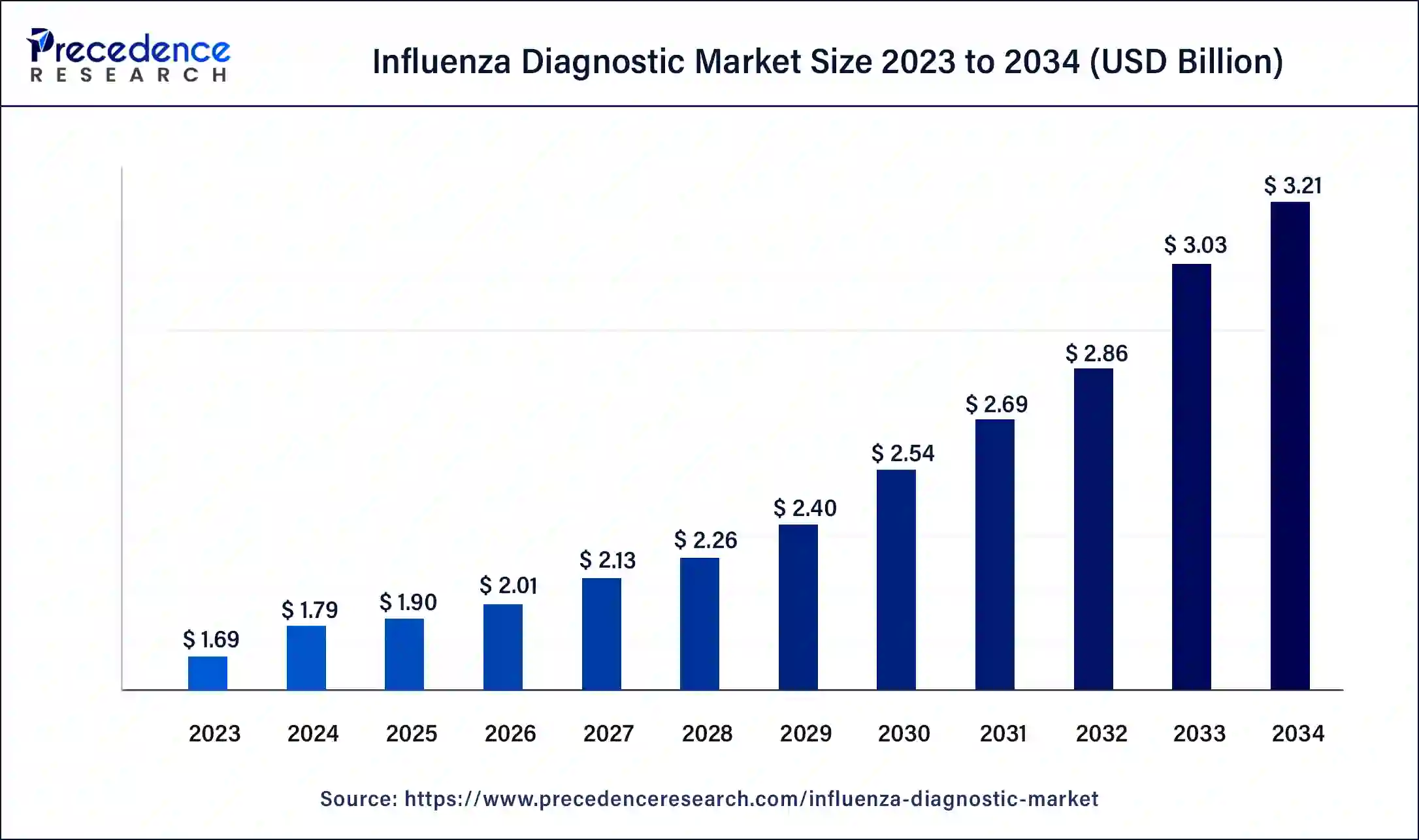
The global influenza diagnostic market size is predicted to hit around USD 2.97 billion by 2033, growing at a CAGR of 5.80% from 2024 to 2033.
Key Points
- North America dominated the market with the highest market share of 34% in 2023.
- Asia Pacific is observed to be the fastest-growing region in the market during the forecast period.
- By test type, the RIDT segment has contributed the largest share of 31% in 2023.
- By end-use, the hospitals segment dominated the market with the major market share of 48% in 2023.
The influenza diagnostic market is a segment within the broader healthcare industry that focuses on the development, production, and distribution of diagnostic tests for influenza viruses. Influenza, commonly known as the flu, is a highly contagious respiratory illness caused by influenza viruses. The market for influenza diagnostics encompasses various types of tests, including rapid antigen tests, molecular tests (such as PCR), and serological tests, aimed at detecting the presence of influenza virus antigens or antibodies in patient samples. These tests play a crucial role in the timely and accurate diagnosis of influenza, enabling healthcare providers to initiate appropriate treatment and implement preventive measures to control outbreaks.
Get a Sample: https://www.precedenceresearch.com/sample/3965
Growth Factors:
Several factors contribute to the growth of the influenza diagnostic market. Firstly, the increasing prevalence of influenza outbreaks, particularly during seasonal peaks, drives the demand for rapid and accurate diagnostic tests. Additionally, the growing awareness among healthcare professionals and the general population about the importance of early diagnosis and prompt treatment of influenza further fuels market growth. Moreover, advancements in diagnostic technologies, such as the development of molecular assays with improved sensitivity and specificity, enhance the reliability of influenza diagnostics, driving adoption rates. Furthermore, the rising investments in healthcare infrastructure, especially in developing regions, and the expanding access to diagnostic facilities contribute to market expansion.
Region Insights:
The influenza diagnostic market exhibits regional variations influenced by factors such as healthcare infrastructure, epidemiological trends, regulatory frameworks, and economic conditions. Developed regions like North America and Europe hold significant market shares due to their well-established healthcare systems, high adoption rates of advanced diagnostic technologies, and proactive measures to control influenza outbreaks. In these regions, stringent regulations regarding disease surveillance and diagnostic testing drive market growth. Conversely, emerging economies in Asia-Pacific and Latin America are witnessing rapid market expansion attributed to increasing healthcare expenditure, growing awareness about infectious diseases, and improving access to diagnostic services.
Influenza Diagnostic Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 5.80% |
| Global Market Size in 2023 | USD 1.69 Billion |
| Global Market Size by 2033 | USD 2.97 Billion |
| U.S. Market Size in 2023 | USD 400 Million |
| U.S. Market Size by 2033 | USD 710 Million |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Test Type and By End-use |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Influenza Diagnostic Market Dynamics
Drivers:
Several drivers propel the growth of the influenza diagnostic market. Firstly, the seasonal nature of influenza outbreaks, coupled with the potential for pandemics, underscores the need for robust diagnostic strategies to facilitate timely interventions and prevent the spread of the virus. Additionally, the growing geriatric population, who are more susceptible to influenza-related complications, drives demand for diagnostic tests in healthcare settings catering to elderly patients. Furthermore, the increasing focus on point-of-care testing and the development of portable, user-friendly diagnostic devices enable rapid and convenient detection of influenza viruses, driving market growth. Moreover, initiatives by governments and healthcare organizations to enhance influenza surveillance and promote vaccination campaigns stimulate market expansion.
Opportunities:
The influenza diagnostic market presents several opportunities for stakeholders to capitalize on emerging trends and technological advancements. Firstly, the integration of artificial intelligence (AI) and machine learning algorithms into diagnostic platforms holds the potential to enhance the accuracy and efficiency of influenza detection, paving the way for the development of next-generation diagnostic solutions. Additionally, the growing emphasis on personalized medicine and precision diagnostics creates opportunities for the customization of influenza diagnostic tests based on individual patient characteristics and viral strains. Furthermore, collaborations between healthcare organizations, research institutions, and industry players can facilitate the development of innovative diagnostic technologies and expand market reach across diverse geographies.
Challenges:
Despite the promising growth prospects, the influenza diagnostic market faces several challenges that warrant attention. Firstly, the emergence of new influenza virus strains and the constant evolution of existing strains pose challenges for diagnostic test manufacturers in ensuring the accuracy and effectiveness of their products. Additionally, regulatory hurdles and compliance requirements vary across different regions, leading to complexities in market entry and product approvals. Furthermore, the competition from alternative diagnostic methods, such as clinical assessment and syndromic surveillance, presents challenges for market penetration and adoption. Moreover, the economic constraints in certain regions, coupled with the high cost of advanced diagnostic technologies, can hinder market growth and accessibility, particularly in resource-limited settings.
Read Also: Mammography Systems Market Size to Rake USD 6.22 Bn by 2033
Recent Developments
- In May 2023, Hologic Inc. declared that the Panther Fusion assay had received 510(k) clearance from the U.S. Food and Drug Administration (FDA). This assay is a molecular diagnostic test that can identify and distinguish between four common respiratory viruses that can present with similar clinical symptoms: influenza A (flu A), influenza B (flu B), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and respiratory syncytial virus (RSV).
- In January 2023, the healthcare goods and solutions firm 2San released a dual kit for SARS-CoV-2 and Influenza A+B. The OTC kit was introduced to ease the strain on healthcare facilities due to the continued demand on the National Health Service (NHS) following the epidemic.
Influenza Diagnostic Market Companies
- 3M Company
- Abbott Laboratories
- Becton, Dickinson and Company (BD)
- Meridian Bioscience, Inc.
- Quidel Corporation
- SEKISUI Diagnostics
- Thermo Fisher Scientific, Inc.
- Hologic, Inc.
- F. Hoffmann-La Roche Ltd
- SA Scientific Ltd
Segments Covered in the Report
By Test Type
- RIDT
- RT-PCR
- Cell Culture
- Others
By End-use
- Hospitals
- POCT
- Laboratories
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/