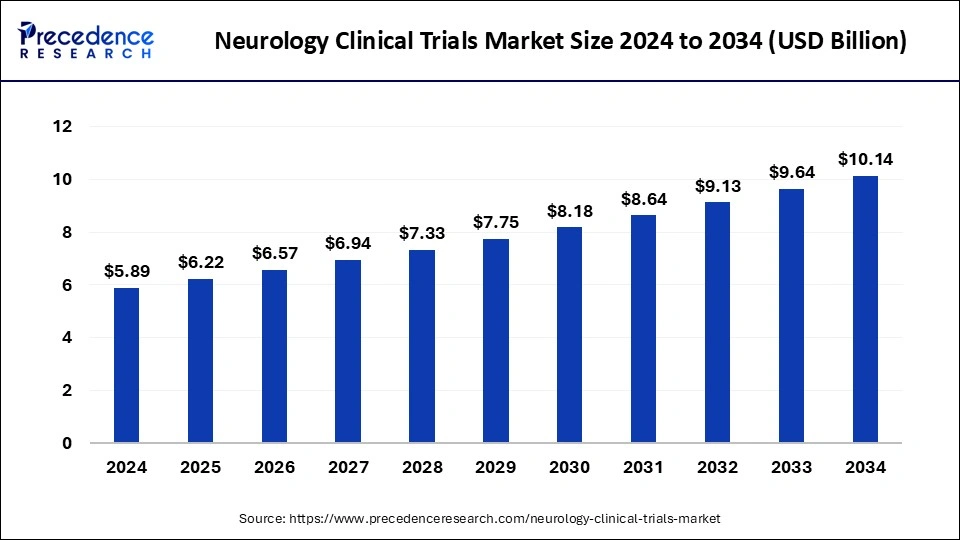
The global neurology clinical trials market size was estimated at USD 5.58 billion in 2023 and is predicted to be worth around USD 9.64 billion by 2033, expanding at a CAGR of 5.62% from 2024 to 2033.
Neurology Clinical Trials Market Key Points
- North America dominated the neurology clinical trials market with the largest revenue share of 48% in 2023.
- Asia Pacific is estimated to grow at a solid CAGR of 5.93% during the forecast period of 2024-2033.
- By phase, the phase II segment has generated more than 47% of revenue share in 2023.
- By phase, the phase III segment is projected to expand at a CAGR of 5.62% during the forecast period.
- By indication, the epilepsy segment has recorded more than 23% of revenue share in 2023.
- By indication, the huntington’s disease segment growing at a CAGR of 6.04% during the forecast period.
- By study design, the interventional segment dominated the market with the biggest revenue share of 96% in 2023.
- By study design, the observational segment is expected to grow at a CAGR of 5.83% during the forecast period.
The neurology clinical trials market plays a crucial role in advancing treatments for various neurological disorders, which encompass a wide range of conditions affecting the brain, spinal cord, and nervous system. These trials are essential for evaluating new therapies, improving existing treatments, and understanding disease mechanisms. Neurological disorders present significant challenges due to their complex nature and varying degrees of severity, necessitating rigorous clinical research to develop effective interventions.
Regional Insights
The market for neurology clinical trials exhibits regional variations influenced by healthcare infrastructure, regulatory frameworks, and prevalence of neurological disorders. North America holds a prominent position, driven by robust research capabilities, high healthcare expenditure, and supportive regulatory environments such as the FDA’s expedited pathways for neurological therapies. Europe follows closely, benefiting from extensive academic and research institutions focusing on neurology. Emerging economies in Asia-Pacific are increasingly contributing to the market growth, spurred by rising healthcare investments and a growing patient pool.
Neurology Clinical Trials Market Trends
- Increasing Incidence of Neurological Disorders: As neurological disorders such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis become more prevalent globally, the demand for clinical trials to develop effective treatments and therapies continues to rise.
- Advancements in Neuroimaging and Biomarkers: The integration of advanced neuroimaging techniques and biomarkers in clinical trials has revolutionized diagnosis, patient stratification, and monitoring of treatment efficacy. This trend enhances trial efficiency and accuracy in evaluating neurological treatments.
- Rise of Personalized Medicine: The shift towards personalized medicine, driven by advancements in genetics and molecular biology, is influencing neurology clinical trials. Trials are increasingly focusing on patient-specific treatments based on genetic profiles and biomarker analysis.
- Growing Adoption of Digital Health Technologies: Digital health technologies, including wearable devices, mobile health apps, and telemedicine platforms, are being integrated into neurology clinical trials. These technologies enable remote monitoring of patients, real-time data collection, and enhance patient engagement.
- Expansion of Global Clinical Trial Infrastructure: Emerging markets are becoming increasingly important for conducting neurology clinical trials due to lower costs, large patient pools, and evolving regulatory frameworks. This trend is facilitating more diverse and inclusive trial populations.
Market Scope
| Report Coverage | Details |
| Market Size by 2033 | USD 9.64 Billion |
| Market Size in 2023 | USD 5.58 Billion |
| Market Size in 2024 | USD 5.89 Billion |
| Market Growth Rate from 2024 to 2033 | CAGR of 5.62% |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Phase, Study Design, Indication, Study Design, Phase, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Market Dynamics
Drivers of Market Growth
Several factors drive growth in the neurology clinical trials market. Advances in neuroimaging technologies and biomarker identification enhance diagnostic precision and trial outcomes. Increasing prevalence of neurological disorders globally, including Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis, fuels demand for new treatments. Moreover, collaborations between pharmaceutical companies, research institutions, and patient advocacy groups facilitate innovative trial designs and patient recruitment strategies. The shift towards personalized medicine and targeted therapies also propels the demand for specialized neurological clinical trials.
Opportunities for Expansion
Opportunities abound in expanding the neurology clinical trials market. Rapid adoption of digital health technologies such as telemedicine and wearable devices streamlines data collection, improves patient monitoring, and enhances trial efficiency. Moreover, regulatory agencies’ emphasis on patient-centric approaches and real-world evidence broadens the scope for diverse trial designs and data sources. Collaborative initiatives across global research networks enable multi-center trials, leveraging diverse patient populations and accelerating trial timelines. Furthermore, increasing investments in orphan drug development for rare neurological disorders present niche opportunities for clinical research.
Challenges Facing the Market
Despite growth prospects, the neurology clinical trials market faces several challenges. High costs associated with neurological research, including specialized imaging and stringent regulatory requirements, pose financial barriers, particularly for small biotech firms and academic researchers. Complexities in trial recruitment and retention, exacerbated by the heterogeneous nature of neurological diseases, challenge timely completion of trials. Additionally, variability in disease progression and response to treatment necessitates adaptive trial designs, adding complexity and potentially extending trial durations. Addressing these challenges requires innovative approaches, stakeholder collaboration, and adaptive strategies tailored to the unique characteristics of neurological disorders.
Read Also: Menstrual Health Apps Market Size to Worth USD 9.04 Bn by 2033
Recent Developments
- In August 2023, the phase II RECOVER-NEURO clinical trial study to evaluate the combination of REMOTE-transcranial direct current stimulation (tDCS) and a brain training program for long covid was launched by Soterix Medical.
- In March 2024, an innovative and new pTau217 blood test, ALZpath Dx, was launched by a specialized clinical laboratory, Neurocode USA, Inc., that offers world-class testing solutions for neurological disorders. This new test may be used in the monitoring, screening, and diagnosis of Alzheimer’s disease. In the US, Neurocode is the first laboratory to make this test as LDT (laboratory-developed test) for clinical trials, clinical diagnostics use, and other research causes.
Neurology Clinical Trials Market Companies
- IQVIA
- Biogen
- Aurora Healthcare
- GlaxoSmithKline Plc.
- Icon Plc.
- Syneous Health
- Charles River Laboratories
- Med pace
- Covance
- Novartis AG
- Sanofi
- Merck & Co., Inc.
- AbbVie Inc.
- Teva Pharmaceutical Industries Ltd.
- Annovis Bio
- Athira Pharma, Inc.
- Zydus Group
- Eli Lilly and Company
- Eisai Co., Ltd.
- AstraZeneca
- Supernus Pharmaceuticals, Inc. (Adamas Pharmaceuticals)
Segments Covered in the Report
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design
- Interventional
- Observational
- Expanded Access
By Indication
- Epilepsy
- Parkinson’s Disease (PD)
- Huntington’s Disease
- Stroke
- Traumatic Brain Injury (TBI)
- Amyotrophic Lateral Sclerosis (ALS)
- Muscle Regeneration
- Others
By Study Design
- Epilepsy
- Interventional
- Observational
- Expanded Access
- Parkinson’s Disease (PD)
- Interventional
- Observational
- Expanded Access
- Huntington’s Disease
- Interventional
- Observational
- Expanded Access
- Stroke
- Interventional
- Observational
- Expanded Access
- Traumatic Brain Injury (TBI)
- Interventional
- Observational
- Expanded Access
- Amyotrophic Lateral Sclerosis (ALS)
- Interventional
- Observational
- Expanded Access
- Muscle Regeneration
- Interventional
- Observational
- Expanded Access
- Others
- Interventional
- Observational
- Expanded Access
by Phase
- Epilepsy
- Phase I
- Phase II
- Phase III
- Phase IV
- Parkinson’s Disease (PD)
- Phase I
- Phase II
- Phase III
- Phase IV
- Huntington’s Disease
- Phase I
- Phase II
- Phase III
- Phase IV
- Stroke
- Phase I
- Phase II
- Phase III
- Phase IV
- Traumatic Brain Injury (TBI)
- Phase I
- Phase II
- Phase III
- Phase IV
- Amyotrophic Lateral Sclerosis (ALS)
- Phase I
- Phase II
- Phase III
- Phase IV
- Muscle Regeneration
- Phase I
- Phase II
- Phase III
- Phase IV
- Others
- Phase I
- Phase II
- Phase III
- Phase IV
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/


