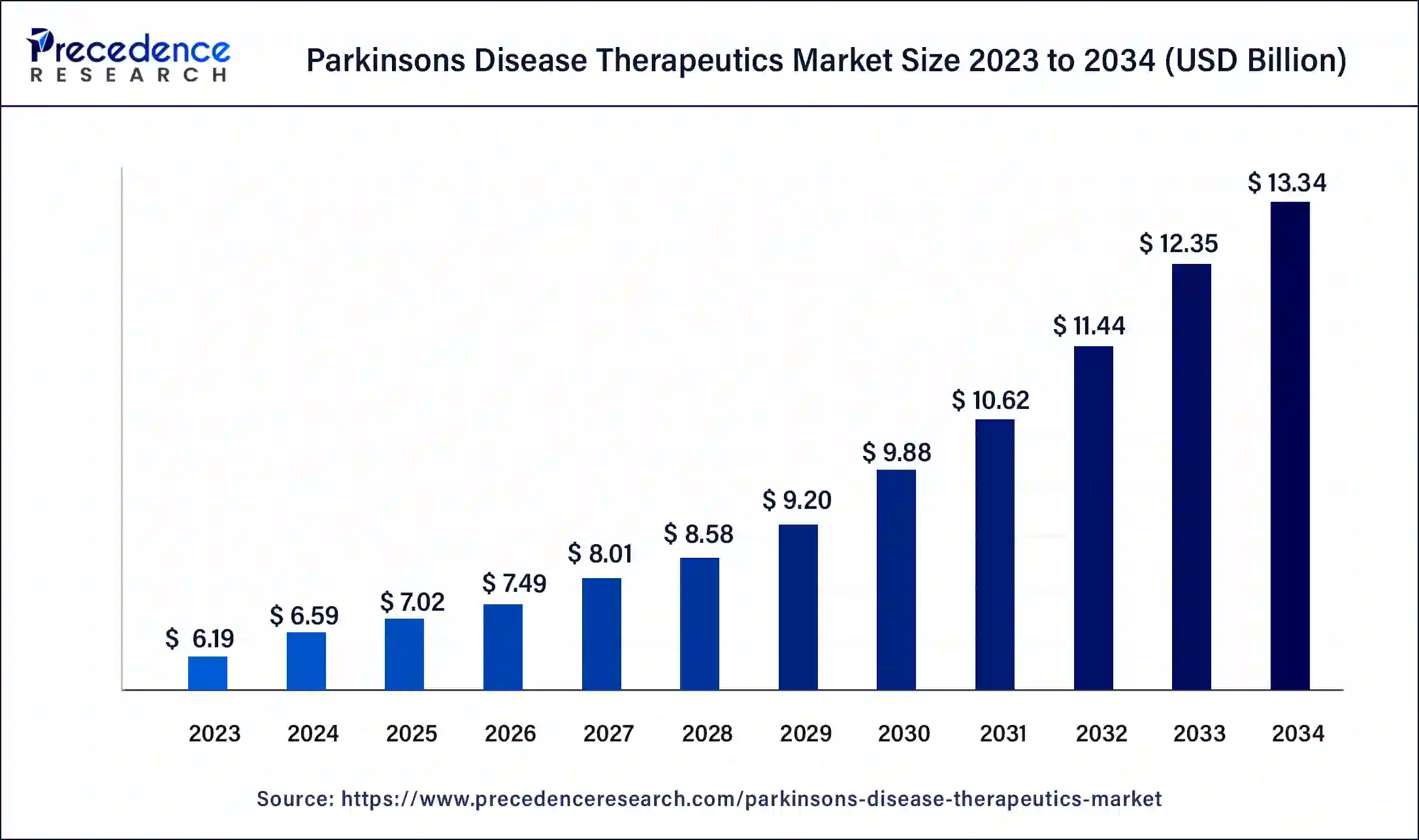
The global parkinson’s disease therapeutics market size was valued at USD 5.90 billion in 2023 and is expected to reach around USD 11.08 billion by 2033, expanding at a CAGR of 6.51% from 2024 to 2033.
Key Points
- The North America parkinson’s disease therapeutics market size accounted for USD 2.36 billion in 2023 and is expected to expand around USD 4.49 billion by 2033, at a CAGR of 6.64% from 2024 to 2033.
- North America dominated the market with the largest revenue share of 40% in 2023.
- Europe is expected to grow to the highest CAGR during the forecast period.
- By drug class, the levodopa/carbidopa segment dominated the market in 2023.
- By drug class, the COMT inhibitors segment is expected to grow at the fastest CAGR during the forecast period.
- By route of administration, the oral segment has contributed more than 67% of revenue share in 2023.
- By patient, the adult segment has held a major revenue share of 85% in 2023.
Parkinson’s disease (PD) is a chronic and progressive neurological disorder characterized by motor symptoms such as tremors, rigidity, bradykinesia, and postural instability, as well as non-motor symptoms including cognitive impairment, mood disorders, and autonomic dysfunction. The pathophysiology of PD primarily involves the degeneration of dopaminergic neurons in the substantia nigra, leading to a deficit in dopamine, a neurotransmitter crucial for regulating movement. The global Parkinson’s Disease Therapeutics market encompasses a range of treatment modalities aimed at managing symptoms and improving quality of life, as there is currently no cure for the disease.
The therapeutic landscape for Parkinson’s disease includes medications such as levodopa, dopamine agonists, MAO-B inhibitors, and COMT inhibitors, as well as non-pharmacological treatments like deep brain stimulation (DBS) and physical therapy. Additionally, emerging therapies, including gene therapy and stem cell therapy, are being explored to offer more effective and potentially disease-modifying options. The market for Parkinson’s disease therapeutics is driven by the increasing prevalence of the disease, advances in research, and the development of innovative treatments.
Get a Sample: https://www.precedenceresearch.com/sample/4430
Growth Factors
The growth of the Parkinson’s Disease Therapeutics market is fueled by several key factors. First and foremost is the rising prevalence of Parkinson’s disease worldwide, which is largely attributed to an aging global population. As life expectancy increases, the incidence of age-related diseases like Parkinson’s also rises, creating a larger patient population in need of therapeutic interventions.
Advancements in medical research and technology have led to a deeper understanding of the disease’s mechanisms, which in turn has facilitated the development of novel therapeutics. The advent of precision medicine and personalized treatment approaches is also contributing to market growth, as these strategies aim to tailor treatments to individual patients based on genetic, biomarker, and phenotypic information, potentially improving outcomes.
Furthermore, increased investment in research and development by pharmaceutical companies, coupled with supportive government initiatives and funding, is accelerating the discovery and commercialization of new treatments. The growing interest in neurodegenerative diseases from both the public and private sectors is fostering a robust pipeline of potential therapies.
Region Insights
The Parkinson’s Disease Therapeutics market exhibits significant regional variation, driven by differences in healthcare infrastructure, disease prevalence, and economic factors. North America holds a substantial share of the market, primarily due to its well-established healthcare system, high healthcare expenditure, and strong presence of key market players. The United States, in particular, is a major contributor to market growth, with extensive research activities and numerous clinical trials aimed at discovering new treatments.
Europe is another significant market, with countries like Germany, the United Kingdom, and France leading the way. The region benefits from a combination of advanced healthcare systems, high awareness about Parkinson’s disease, and substantial funding for neurological research. The European Medicines Agency (EMA) also plays a critical role in the approval and regulation of new therapeutics, ensuring that innovative treatments reach patients efficiently.
In the Asia-Pacific region, the market is growing rapidly due to increasing healthcare awareness, rising disposable incomes, and improving access to medical care. Countries such as China, Japan, and India are witnessing a surge in Parkinson’s disease cases, driven by demographic shifts and lifestyle changes. The region’s burgeoning pharmaceutical industry is also contributing to market growth, with several companies actively engaged in the development of PD therapeutics.
Latin America and the Middle East & Africa are emerging markets for Parkinson’s disease therapeutics. While these regions currently face challenges such as limited healthcare infrastructure and lower economic development, ongoing efforts to improve healthcare access and increased focus on neurological disorders are expected to drive future growth.
Parkinson’s Disease Therapeutics Market Scope
| Report Coverage | Details |
| Market Size in 2023 | USD 5.90 Billion |
| Market Size in 2024 | USD 6.28 Billion |
| Market Size by 2033 | USD 11.08 Billion |
| Market Growth Rate | CAGR of 6.51% from 2024 to 2033 |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Drug Class, Route of Administration, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Parkinson’s Disease Therapeutics Market Dynamics
Drivers
Several factors are driving the growth of the Parkinson’s Disease Therapeutics market. One of the primary drivers is the increasing prevalence of Parkinson’s disease, which is expected to rise further with the aging global population. The growing awareness about the disease and its symptoms is leading to earlier diagnosis and treatment, which in turn is boosting the demand for therapeutics.
Technological advancements in medical research and the development of new treatment modalities are also significant drivers. Innovations such as deep brain stimulation (DBS), gene therapy, and stem cell therapy offer new hope for patients and are expanding the therapeutic landscape. These cutting-edge treatments, combined with improvements in existing medications, are enhancing the efficacy and safety of Parkinson’s disease management.
The supportive regulatory environment and favorable government initiatives are further propelling market growth. Regulatory agencies like the FDA and EMA are prioritizing the approval of novel therapies for neurodegenerative diseases, streamlining the process for bringing new treatments to market. Additionally, government funding for Parkinson’s disease research is increasing, encouraging the development of innovative therapeutics.
The rise of personalized medicine and the growing emphasis on patient-centric care are also driving market growth. Personalized treatment approaches, which take into account individual genetic and phenotypic profiles, are being increasingly adopted to optimize therapeutic outcomes and minimize adverse effects. This trend is supported by advancements in diagnostic technologies and the growing availability of genetic testing.
Opportunities
The Parkinson’s Disease Therapeutics market presents numerous opportunities for growth and innovation. One of the most promising areas is the development of disease-modifying therapies that go beyond symptom management to address the underlying causes of Parkinson’s disease. Advances in genetic research and molecular biology are paving the way for targeted therapies that could potentially slow or halt disease progression.
Gene therapy is one such area with immense potential. By delivering genetic material directly into patients’ cells, gene therapy aims to correct or replace defective genes responsible for Parkinson’s disease. Several gene therapy candidates are currently in clinical trials, showing promising results in preclinical studies. If successful, these therapies could revolutionize the treatment of Parkinson’s disease and significantly improve patient outcomes.
Another area of opportunity is the use of biomarkers for early diagnosis and treatment monitoring. Biomarkers can provide valuable insights into disease progression and treatment response, enabling more precise and timely interventions. The identification and validation of reliable biomarkers for Parkinson’s disease are ongoing, with several research initiatives focused on this goal.
The expansion of telemedicine and digital health technologies also offers new avenues for improving patient care. Remote monitoring and telehealth services can enhance access to medical care, especially for patients in underserved or remote areas. Digital health platforms can facilitate better disease management, medication adherence, and patient engagement, ultimately leading to improved outcomes.
Challenges
Despite the promising growth prospects, the Parkinson’s Disease Therapeutics market faces several challenges. One of the most significant challenges is the complexity of the disease itself. Parkinson’s disease is a multifaceted disorder with a wide range of symptoms and variations in disease progression among patients. This heterogeneity makes it difficult to develop universal treatments and requires a tailored approach to therapy.
The lack of a definitive cure for Parkinson’s disease is another major challenge. Current treatments primarily focus on managing symptoms, and while they can improve quality of life, they do not address the underlying neurodegeneration. The development of disease-modifying therapies is a critical unmet need, but it is a complex and time-consuming process that requires significant investment in research and clinical trials.
The high cost of treatment is also a barrier to market growth. Advanced therapies such as deep brain stimulation (DBS) and novel drug formulations can be expensive, limiting their accessibility to patients, especially in low- and middle-income countries. Efforts to reduce the cost of treatment and improve affordability are essential for broader market penetration.
Regulatory hurdles and the lengthy approval process for new therapies can also impede market growth. While regulatory agencies are increasingly prioritizing neurodegenerative diseases, the stringent requirements for clinical trials and safety evaluations can delay the introduction of new treatments. Ensuring a balance between rigorous safety standards and timely access to innovative therapies is a key challenge for the industry.
Finally, the need for more comprehensive and inclusive clinical trials is a challenge that needs to be addressed. Clinical trials for Parkinson’s disease often have stringent inclusion criteria, which can limit the diversity of patient populations and affect the generalizability of results. There is a growing recognition of the need for more inclusive trial designs that reflect the real-world patient population.
Parkinson’s Disease Therapeutics Market Companies
- Cerevel Therapeutics
- Hallamshire Physiotherapy Ltd
- Novartis AG
- Olatec Therapeutics
- Biotech Ltd
- Teva Pharmaceutical Industries Ltd.
- Merck & Co., Inc.
- GlaxoSmithKline plc. (GSK)
- AbbVie, Inc.
- Acorda Therapeutics, Inc.
- H. Lundbeck A/S
- Amneal Pharmaceuticals LLC
- Supernus Pharmaceuticals, Inc.
Parkinson’s Disease Therapeutics Market Recent Developments
- In February 2024, Hallamshire Physiotherapy Ltd, Sheffield, launched a revolutionary new therapy to help people with Parkinson’s manage some of their symptoms. Stroll Augmented Reality Therapy involves patients wearing a special headset linked to specialist digital therapeutic software to help improve mobility, confidence, and independence.
- In January 2024, AbbVie announced the launch of Produodopa in Europe. Produodopa is useful for the treatment of advanced Parkinson’s disease with severe motor fluctuations and hyperkinesia or dyskinesia.
- In August 2023, Acorda Therapeutics, Inc. announced the launch of a new INBRIJA (levodopa inhalation powder) website and brand campaign. The campaign, “For the Fighters,” is based on direct feedback from people with Parkinson’s (PwPs).
Segments Covered in the Report
By Drug Class
- Levodopa/Carbidopa
- Dopamine Agonists
- Adenosine A2A Antagonist
- COMT Inhibitors
- MAO-B Inhibitors
- Glutamate Antagonist
- Others
By Route of Administration
- Oral
- Subcutaneous
- Transdermal
- Others
By Patient
- Adult
- Pediatric
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/