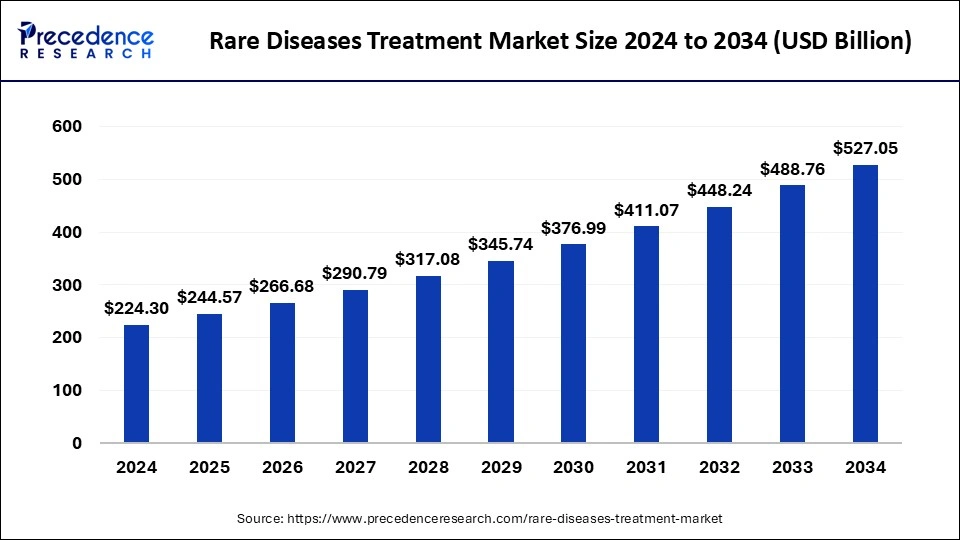
The global rare diseases treatment market size was estimated at USD 205.70 billion in 2023 and is projected to be worth around USD 488.76 billion by 2033, growing at a CAGR of 9.04% from 2024 to 2033.
Key Points
- North America dominated the market with the biggest revenue share of 49% in 2023.
- Asia Pacific is expected to grow at the highest CAGR in the market during the forecast period.
- By drug type, the biologics segment has contributed more than 82% of revenue share in 2023.
- By drug type, the non-biologic segment is expected to grow rapidly in the market during the forecast period.
- By therapeutic area, the cancer segment has held a major revenue share of 50% in 2023.
- By therapeutic area, the blood-related disorder segment is expected to grow at a significant CAGR in the market during the forecast period.
- By route of administration, the injectable segment dominated the market in 2023.
- By route of administration, the oral segment is expected to grow at the highest CAGR in the market during the forecast period.
The Rare Diseases Treatment Market encompasses medical interventions, therapies, and pharmaceuticals specifically developed to address diseases that affect a small percentage of the population. These conditions, often referred to as orphan diseases, pose unique challenges due to their rarity, which can include limited understanding, diagnostic difficulties, and scarcity of treatment options. Despite these challenges, the market has seen significant growth driven by advancements in research, increasing awareness, and supportive regulatory initiatives.
Get a Sample: https://www.precedenceresearch.com/sample/4410
Table of Contents
ToggleGrowth Factors
Several factors contribute to the growth of the Rare Diseases Treatment Market. Technological advancements in genomics, molecular biology, and biotechnology have facilitated better understanding of rare diseases, enabling the development of targeted therapies. Additionally, rising investments in research and development, along with government incentives such as orphan drug designation and market exclusivity, have encouraged pharmaceutical companies to invest in developing treatments for rare diseases.
Region Insights:
The Rare Diseases Treatment Market exhibits regional variations influenced by factors such as healthcare infrastructure, regulatory frameworks, and prevalence of rare diseases. Developed regions like North America and Europe lead in terms of market share, owing to established healthcare systems, robust research infrastructure, and high awareness levels. However, emerging economies in Asia-Pacific and Latin America are witnessing rapid growth, driven by improving healthcare access, increasing disposable income, and growing investment in healthcare infrastructure.
Rare Diseases Treatment Market Scope
| Report Coverage | Details |
| Rare Diseases Treatment Market Size in 2023 | USD 205.70 Billion |
| Rare Diseases Treatment Market Size in 2024 | USD 224.30 Billion |
| Rare Diseases Treatment Market Size by 2033 | USD 488.76 Billion |
| Rare Diseases Treatment Market Growth Rate | CAGR of 9.04% from 2024 to 2033 |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Drug Type, Therapeutic Area, Patient, Route of Administration, Distribution Channel, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Rare Diseases Treatment Market Dynamics
Drivers:
Key drivers of the Rare Diseases Treatment Market include the growing prevalence of rare diseases, fueled by factors such as genetic mutations, environmental factors, and better diagnostic capabilities. Additionally, increasing public-private partnerships, rising demand for personalized medicine, and expanding orphan drug development programs are driving market growth. Moreover, advancements in precision medicine and gene therapy hold promise for treating previously untreatable rare diseases, further stimulating market expansion.
Opportunities
The Rare Diseases Treatment Market presents numerous opportunities for stakeholders. Collaboration among pharmaceutical companies, academic institutions, and regulatory agencies can accelerate drug discovery and development processes. Moreover, expanding patient advocacy efforts and increasing patient engagement in research can enhance understanding of rare diseases and facilitate the development of patient-centric treatments. Furthermore, the adoption of innovative technologies such as artificial intelligence and big data analytics offers opportunities for improving diagnosis, treatment monitoring, and patient outcomes.
Challenges
Despite the growth prospects, the Rare Diseases Treatment Market faces several challenges. Limited understanding of disease mechanisms, coupled with small patient populations, poses challenges in clinical trial design and recruitment. Additionally, high development costs, regulatory hurdles, and reimbursement challenges hinder investment in rare disease research and development. Moreover, disparities in access to healthcare and diagnostic facilities, especially in low- and middle-income countries, exacerbate challenges in diagnosing and treating rare diseases. Addressing these challenges requires concerted efforts from stakeholders across the healthcare ecosystem.
Read Also: Mutual Fund Assets Market Size to Reach USD 1,146.27 Bn by 2033
Rare Diseases Treatment Market Recent Developments
- In May 2024, After a 2023 approval from the FDA, Krystal Biotech collected more than $95 million from its launch of Vyjuvek, the first treatment for the rare skin disease dystrophic epidermolysis bullosa (DEB).
- In February 2024, Zydus Lifesciences announced the launch of its first new drug in the United States by early 2026, looking to tap into the multi-billion dollar market for treating a type of liver disease, Managing Director Sharvil Patel told Reuters.
- In February 2024, Florida State University launched the Institute for Pediatric Rare Diseases, an institute dedicated to advancing research and developing treatments for unusual childhood diseases, filling a critical gap in the healthcare industry. The institute is made possible by $1 million in funding from the Florida Legislature.
Rare Diseases Treatment Market Companies
- F. Hoffmann-La Roche Ltd.
- Pfizer, Inc.
- PTC Therapeutics
- AstraZeneca
- Novartis AG
- Takeda Pharmaceutical Company
- Bayer AG
- AbbVie Inc.
- Merck & Co. Inc.
- Bristol Myers Squibb
Segment Covered in the Report
By Drug Type
- Biologics
- Non-biologics
By Therapeutic Area
- Cancer
- Blood-Related Disorder
- Central Nervous System
- Respiratory Disorders
- Musculoskeletal Disorders
- Cardiovascular Disorder
- Other Therapeutic Areas
By Patient
- Adult
- Pediatric
By Route of Administration
- Oral
- Injectable
- Others
By Distribution Channel
- Hospital Pharmacy
- Specialty Pharmacy
- Online Pharmacy
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/