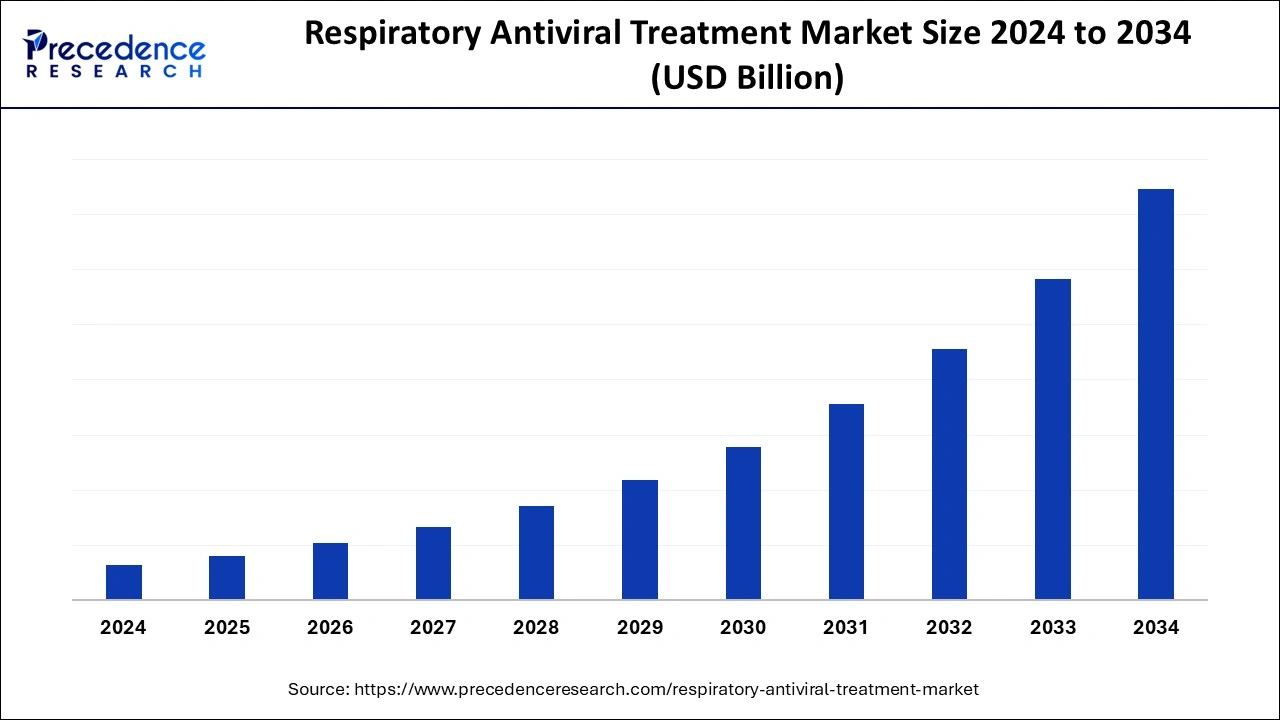
The global respiratory antiviral treatment market is surging, with an overall revenue growth expectation of hundreds of millions of dollars from 2023 to 2032. The rising prevalence of heart disease sector across the world is driving the growth of the respiratory antiviral treatment market.
Key Points
- By region, North America led the respiratory antiviral treatment market with the largest share in 2023.
- By region, Asia Pacific is expected to estimate to be the fastest rate of growth during the forecast period.
- By drug class, the neuraminidase inhibitors segment led the market in 2023.
- By drug class, the nucleoside analogs segment is expected to be the fastest growing segment during the forecast period.
- By disease type, the influenza segment dominated the market in 2023.
- By distribution channel, the hospital pharmacy segment dominated the respiratory antiviral treatment market in 2023.
- By distribution channel, the retail pharmacy segment is estimated to grow with the significant CAGR during the forecast period.
The respiratory antiviral treatment market is focused on pharmaceuticals and therapies designed to combat viral infections affecting the respiratory system. These treatments are crucial in managing diseases such as influenza, respiratory syncytial virus (RSV), and COVID-19, among others. The market encompasses a range of antiviral drugs and therapies aimed at alleviating symptoms, reducing severity, and shortening the duration of respiratory viral infections.
Get a Sample: https://www.precedenceresearch.com/sample/4549
Growth Factors
The market for respiratory antiviral treatments is driven by several key factors. Increasing global incidences of respiratory viral infections, coupled with growing awareness and healthcare infrastructure improvements, contribute significantly to market growth. Advances in medical research and technology continue to enhance the efficacy and availability of antiviral treatments, further fueling market expansion. Additionally, the rise in pandemics and seasonal outbreaks underscores the critical need for effective antiviral therapies, bolstering market demand.
Regional Insights
Regionally, North America and Europe dominate the respiratory antiviral treatment market due to high healthcare expenditures, robust research and development activities, and early adoption of advanced medical technologies. Asia-Pacific is witnessing rapid growth driven by improving healthcare infrastructure, increasing healthcare spending, and rising awareness among the population regarding respiratory infections and their management.
Trends
A notable trend in the respiratory antiviral treatment market is the development of broad-spectrum antiviral drugs capable of targeting multiple respiratory viruses. Personalized medicine approaches and precision therapies tailored to individual viral strains are also emerging trends. Furthermore, there is a growing trend towards combination therapies that enhance efficacy and reduce the likelihood of viral resistance.
Respiratory Antiviral Treatment Market Scope
| Report Coverage | Details |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Disease Type, Drug Class, Distribution Channel, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Respiratory Antiviral Treatment Market Dynamics
Drivers
Key drivers include the continual evolution of respiratory viruses, necessitating ongoing research and development of novel antiviral treatments. Government initiatives and funding to combat infectious diseases, along with collaborations between pharmaceutical companies and research institutions, also drive market growth. Moreover, the increasing geriatric population, who are more susceptible to respiratory infections, contributes to rising demand for effective antiviral treatments.
Opportunities
Opportunities in the respiratory antiviral treatment market lie in expanding product portfolios through innovation and strategic partnerships. Market players can capitalize on unmet medical needs in emerging economies and invest in advanced drug delivery technologies to improve treatment efficacy and patient compliance. Additionally, the growing demand for over-the-counter (OTC) antiviral treatments presents opportunities for market expansion.
Challenges
Despite growth prospects, the respiratory antiviral treatment market faces challenges such as regulatory hurdles in drug approval processes, especially for new and innovative therapies. The emergence of viral mutations and resistance to existing treatments poses challenges in maintaining treatment efficacy over time. Economic constraints and healthcare disparities in certain regions also impact market accessibility and adoption of antiviral therapies.
Read Also: Nanoparticle Contract Manufacturing Market Size, Share, Report 2033
Respiratory Antiviral Treatment Market Companies
- F. Hoffmann-La Roche Ltd.
- Mylan N.V.
- Sanofi
- Pfizer Inc.
- GlaxoSmithKline plc
- Novartis AG
- Merck & Co., Inc.
- Dr. Reddy’s Laboratories Ltd.
- Zydus Cadila
- Johnson & Johnson Private Limited
- Amneal Pharmaceuticals LLC
- AbbVie Inc.
- Alembic Pharmaceuticals Limited
- Lupin
- Gilead Sciences, Inc.
- Cipla Inc.
- Bausch Health Companies Inc.
- Aurobindo Pharma
- Hetero
- Teva Pharmaceutical Industries Ltd.
Recent Developments
- In June 2024, GSK plc announced that the US Food and Drug Administration (FDA) had approved Arexvy vaccine. Arexvy vaccine is used for the prevention of RSV lower respiratory tract disease (LRTD) in adults aged between 50-59 years of age.
- In May 2024, Sanofi announced that the US Food and Drug Administration approved the extension of Dupixent (dupilumab) for three months as an add-on maintenance treatment for several adult patients suffering from uncontrolled chronic obstructive pulmonary disease (COPD).
- In February 2024, Bioxytran launched ProLectin-M. ProLectin-M is an oral antiviral treatment for treatment of COVID-19.
- In February 2024, Takeda announced that the FDA had approved EOHILIA. EOHILIA is an oral antiviral therapy for treating patients of age 11 years and above suffering from eosinophilic esophagitis (EoE).
- In May 2022, the Centre for Drug Design and Discovery (CD3) and KU Leuven’s Rega Institute for Medical Research collaborated with Gilead Sciences, Inc. This collaboration is done to develop new antiviral medications for treating patients suffering from respiratory syncytial virus (RSV) infection.
Segments Covered in the Report
By Disease Type
- Pneumonia
- Influenza
- Bronchiolitis
- Upper Respiratory Tract Infection
- Others
By Drug Class
- Nucleoside Analogs
- Neuraminidase Inhibitors
- Ion Channel Blockers
- Fusion Protein Inhibitors
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
Blog: https://www.dailytechbulletin.com/
Blog: https://www.autoindustrybulletin.com/