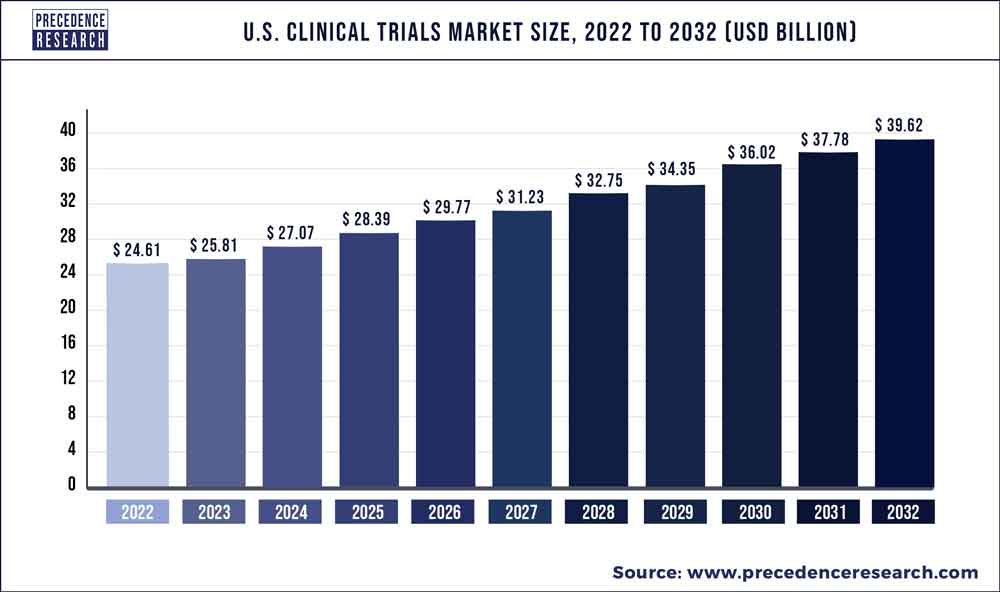
According to the new research report published by Precedence Research, titled “US Clinical Trials Market (By Phase: Phase I, Phase II, Phase III, Phase IV; By Study Design: Interventional, Observational; By Indication: Oncology, Autoimmune, Pain Management, CNS Conditions, Obesity, Cardiovascular, Diabetes) – Industry Analysis, Size, Share, Growth, Trends, and Forecast 2023-2032 (By Product: Traditional, Advanced; By Application: Pottery, Tiles, Abrasives, Sanitary wave, Bricks & pipes, Others; By End User: Medical, Industrial, Building & Construction, Others) – Global Industry Analysis, Size, Share, Growth, Trends, Regional Outlook, and Forecast 2023-2032”, the US clinical trials market size is expected to be worth around US$ 39.62 billion by 2032 and is poised to record a yearly growth rate of 4.88% from 2023 to 2032. The study investigates several elements and their consequences on the growth of the all-wheel drive market.
This report focuses on US clinical trials market volume and value at the global level, regional level and company level. From a global perspective, this report represents the overall US clinical trials market size by analysing historical data and future prospects. Regionally, this report focuses on several key regions: North America, Europe, the Middle East & Africa, Latin America, etc.
The research report includes specific segments by region (country), by company, by all segments. This study provides information about the growth and revenue during the historic and forecasted period of 2017 to 2032. Understanding the segments helps in identifying the importance of different factors that aid the market growth.
Download a Free Copy of Our Latest Sample Report@ https://www.precedenceresearch.com/sample/2480
The study also provides important advancements in organic and inorganic growth strategies in the worldwide US clinical trials market. A lot of corporations are prioritizing new launches, product approvals, and other business expansion techniques. In addition, the report offers profiles of US clinical trials market firms and market strategies. Furthermore, the research focuses on top industry participants, providing information such as company profiles, components and services offered, recent financial data, and key developments.=
US clinical trials Market Report Scope
| Report Coverage | Details |
| Market Size in 2022 | USD 24.61 Billion |
| Market Size by 2032 | USD 39.62 Billion |
| Growth Rate from 2023 to 2032 | CAGR of 4.88% |
| Base Year | 2022 |
| Forecast Period | 2023 to 2032 |
| Segments Covered | By Phase, By Study Design, By Indication |
Also read: US Liquid Biopsy Market Size to Worth US$ 1,816.95 Million by 2032
Market Key Players
Company profiles have been included in the report, which include essentials such as product portfolio, key strategies, along with all-inclusive SWOT analysis on each player. Company presence is mapped and presented through a matrix for all the prominent players, thus providing readers with actionable insights, which helps in thoughtfully presenting market status and predicting the competition level in the US clinical trials market.
Some of the prominent players in the US clinical trials market include
- Parexel International Corp.
- Charles River Laboratory
- PRA Health Sciences
- Wuxi AppTec
- Eli Lilly and Company
- Novo Nordisk A/S
- Clinipace
- Omnicare
- Kendle
- Chiltern
US clinical trials Market Segmentations
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design
- Interventional
- Observational
By Indication
- Oncology
- Autoimmune
- Pain Management
- CNS Conditions
- Obesity
- Cardiovascular
- Diabetes
Report Objectives
- To define, segment, and project the global market size for US clinical trials market
- To understand the structure of the market by identifying its various sub-segments
- To provide detailed information about the key factors influencing the growth of the market (drivers, restraints, opportunities, and industry-specific challenges)
- To analyse the micro-markets, with respect to individual growth trends, future prospects, and their contributions to the total market
- To project the size of the market and its submarkets, in terms of value, with respect to global. (along with their respective key countries)
- To profile key players and comprehensively analyse their core competencies
- To understand the competitive landscape and identify major growth strategies adopted by players across the globe.
- To analyse the competitive developments such as expansions & investments, new product launches, mergers & acquisitions, joint ventures, and agreements
TABLE OF CONTENT
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on US Clinical Trials Market
5.1. COVID-19 Landscape: US Clinical Trials Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. US Clinical Trials Market, By Phase
8.1. US Clinical Trials Market, by Phase, 2023-2032
8.1.1 Phase I
8.1.1.1. Market Revenue and Forecast (2023-2032)
8.1.2. Phase II
8.1.2.1. Market Revenue and Forecast (2023-2032)
8.1.3. Phase III
8.1.3.1. Market Revenue and Forecast (2023-2032)
8.1.4. Phase IV
8.1.4.1. Market Revenue and Forecast (2023-2032)
Chapter 9. US Clinical Trials Market, By Study Design
9.1. US Clinical Trials Market, by Study Design, 2023-2032
9.1.1. Interventional
9.1.1.1. Market Revenue and Forecast (2023-2032)
9.1.2. Observational
9.1.2.1. Market Revenue and Forecast (2023-2032)
Chapter 10. US Clinical Trials Market, By Indication
10.1. US Clinical Trials Market, by Indication, 2023-2032
10.1.1. Oncology
10.1.1.1. Market Revenue and Forecast (2023-2032)
10.1.2. Autoimmune
10.1.2.1. Market Revenue and Forecast (2023-2032)
10.1.3. Pain Management
10.1.3.1. Market Revenue and Forecast (2023-2032)
10.1.4. CNS Conditions
10.1.4.1. Market Revenue and Forecast (2023-2032)
10.1.5. Obesity
10.1.5.1. Market Revenue and Forecast (2023-2032)
10.1.6. Cardiovascular
10.1.6.1. Market Revenue and Forecast (2023-2032)
10.1.7. Diabetes
10.1.7.1. Market Revenue and Forecast (2023-2032)
Chapter 11. Global US Clinical Trials Market, Regional Estimates and Trend Forecast
11.1. U.S.
11.1.1. Market Revenue and Forecast, by Phase (2023-2032)
11.1.2. Market Revenue and Forecast, by Study Design (2023-2032)
11.1.3. Market Revenue and Forecast, by Indication (2023-2032)
Chapter 12. Company Profiles
12.1. Parexel International Corp.
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. Charles River Laboratory
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. PRA Health Sciences
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. Wuxi AppTec
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Eli Lilly and Company
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. Novo Nordisk A/S
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. Clinipace
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. Omnicare
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. Kendle
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. Chiltern
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
Why should you invest in this report?
If you are aiming to enter the global US clinical trials market, this report is a comprehensive guide that provides crystal clear insights into this niche market. All the major application areas for US clinical trials are covered in this report and information is given on the important regions of the world where this market is likely to boom during the forecast period of 2022-2030 so that you can plan your strategies to enter this market accordingly.
Besides, through this report, you can have a complete grasp of the level of competition you will be facing in this hugely competitive market and if you are an established player in this market already, this report will help you gauge the strategies that your competitors have adopted to stay as market leaders in this market. For new entrants to this market, the voluminous data provided in this report is invaluable.
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.pharma-geek.com